The Wilson group solved structures towards the development of an effective HIV vaccine ((Pejchal et al. (2011); McLellan et al. (2011), where the latter paper included significant contributions from Peter Kwong's group using SER CAT at the APS also). In order to protect against infection, a vaccine against HIV-1 must elicit broadly neutralizing antibodies (bnAbs) that are able to recognize a majority of the globally circulating strains. Understanding the epitopes recognized by these bnAbs will improve structure-based immunogen design efforts to optimally present such surfaces to the immune system. The crystal structure of PGT 128 Fab bound to a glycosylated gp120 engineered outer domain containing V3 (eODmV3) reveals a novel, highly exposed epitope located on the heavily glycosylated surface of gp120. The structure gives a detailed view of the epitope recognized by PGT 128 and the somatically related PGT 125-127 bnAbs. The interaction surface is composed of two N-linked glycans (at Asn332 and Asn301 of gp120) and a segment of the V3 loop. High-resolution studies with a Man(nine) ligand further explain the specificity of this class of bnAbs for high-mannose glycans, revealing the many terminal interactions with the D1 and D3 arms of Man(nine). In addition to its significant breadth (~ 72 % of a 160 virus panel), PGT 128 is the most potent naturally elicited bnAb and, thus, there is considerable interest in its mechanism of neutralization. Virus neutralization data indicate that PGT 128 significantly accelerates viral inactivation, an observation that helps explain the one to two orders of magnitude increased potency over that expected based on binding alone.
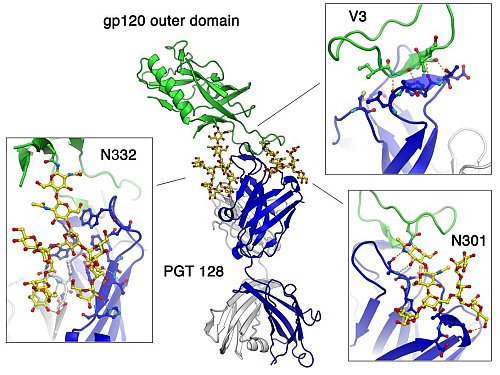 |
Figure: Detailed interactions of broadly neutralizing antibody PGT128 with glycosylated HIV gp120. |
Citation:
| [1] | Pejchal, R, Doores, KJ, Walker, LM, Khayat, R, Huang, PS, Wang, SK, Stanfield, RL, Julien, JP, Ramos, A, Crispin, M, Depetris, R, Katpally, U, Marozsan, A, Cupo, A, Maloveste, S, Liu, Y, McBride, R, Ito, Y, Sanders, RW, Ogohara, C, Paulson, JC, Feizi, T, Scanlan, CN, Wong, CH, Moore, JP, Olson, WC, Ward, AB, Poignard, P, Schief, WR, Burton, DR, Wilson, IA. A Potent and Broad Neutralizing Antibody Recognizes and Penetrates the HIV Glycan Shield, Science 334, 1097-1103 (2011); |
| [2] | McLellan, JS, Pancera, M, Carrico, C, Gorman, J, Julien, JP, Khayat, R, Louder, R, Pejchal, R, Sastry, M, Dai, K, O'Dell, S, Patel, N, Shahzad-ul-Hussan, S, Yang, Y, Zhang, B, Zhou, T, Zhu, J, Boyington, JC, Chuang, GY, Diwanji, D, Georgiev, I, Kwon, YD, Lee, D, Louder, MK, Moquin, S, Schmidt, SD, Yang, ZY, Bonsignori, M, Crump, J A, Kapiga, SH, Sam, NE, Haynes, BF, Burton, DR, Koff, WC, Walker, LM, Phogat, S, Wyatt, R, Orwenyo, J, Wang, LX, Arthos, J, Bewley, CA, Mascola, JR, Nabel, GJ, Schief, WR, Ward, AB, Wilson, IA, Kwong, PD. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9, Nature 480, 336-343 (2011). |