 |
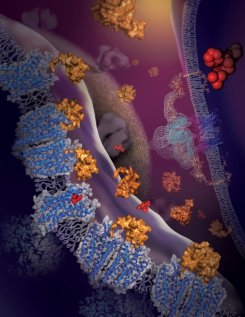
Figure: Schematic of the chemokine receptor in the cellular milieu, including early stages of the HIV-1 entry process in the background [Artwork by Yekaterina Kadyshevskaya, Stevens Laboratory, The Scripps Research Institute]. |
Chemokine receptors are critical regulators of cell migration in the context of immune surveillance, inflammation, and development. The G protein-coupled chemokine receptor CXCR4 is specifically implicated in cancer metastasis and HIV-1 infection. Five independent crystal structures of CXCR4 in complex with a small molecule antagonist IT1t and a cyclic peptide CVX15 at 2.5-to-3.2-Å resolution were solved. As the first structure of protein-activated GPCR, CXCR4 structure differs from other known GPCR structures in helix bundle and ligand binding pocket. Although in multiple crystal forms, all five structures reveal a constant homodimer, which is the first solid observation about a structural GPCR dimer. CXCR4 structures provide new clues about interactions between CXCR4 and its natural chemokine ligand CXCL12, and with HIV-1 glycoprotein gp120. These results have implications for developing new therapeutic strategies for cancer and HIV treatments.
Citation: Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010 Nov 19; 330, 1066-71.