 |
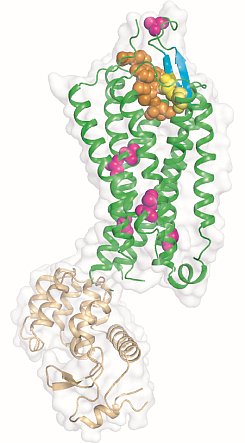
Figure: Overview of the NTS1 structure bound to the peptide agonist NT8-13. Cartoon representation of NTS1 in side view. NTS1 is shown in green and T4 lysozyme used to promote crystal contacts is in brown. Space filling models are used to depict the agonist NTS8-13 (orange), the side chains of thermostabilizing mutations (purple) and the disulphide bond (yellow) between the conserved residues C142 and C225. Also shown are the beta-hairpin in extracellular loop 2 (blue-green) and the pi-helix in intracellular loop 2 (ICL2). |
Reinhard Grisshammer's group at the NIH determined the structure of the agonist-bound neurotensin receptor NTS1, which is the first structure of a G protein-coupled receptor (GPCR) with a bound peptide. Neurotensin (NT) is a 13 amino acid peptide that functions as both a neurotransmitter and as a hormone through activation of the neurotensin receptor NTS1, a GPCR that signals preferentially through Gq. In the brain, NT modulates activity of dopaminergic systems, opioid-independent analgesia, and the inhibition of food intake, and in the gut NT regulates a range of digestive processes. The researchers reported a 2.8-Å structure of NTS1 in an active-like state, bound to NT8-13, the C terminal portion of NT responsible for agonist-induced activation of the receptor. Because wild-type NTS1 is unstable and thus not amenable to crystallization, they used alanine-scanning mutagenesis to stabilize NTS1 and to select for an active-like conformation in the presence of agonist, which combined with the bacteriophage T4 lysozyme fusion protein strategy and the lipidic mesophase crystallization method, resulted in diffracting crystals. The agonist binding pocket is located at the extracellular receptor surface. The peptide agonist binds to NTS1 in an extended conformation nearly perpendicular to the membrane plane with the C-terminus oriented towards the receptor core. The NTS1 structure bears many hallmark features of an active-like receptor conformation such as an outward-tilted transmembrane helix 6 at the cytoplasmic surface and key conserved residues in positions characteristic of active but not inactive GPCRs.
Citation:
White, JF, Noinaj, N, Shibata, Y, Love, J, Kloss, B, Xu, F,
Gvozdenovic-Jeremic, J, Shah, P, Shiloach, J, Tate, CG, Grisshammer, R.
Structure of the agonist-bound neurotensin receptor, Nature 490, 508-513
(2012). DOI: 10.1038/nature11558