Rod MacKinnon's group at the Rockefeller University solved the structure of the human two-pore domain potassium ion channel TRAAK. Potassium-selective ion channels called TRAAK are expressed predominantly in the brain and nervous system where they control cellular electrical excitability. These channels are exquisitely sensitive to the chemical and mechanical properties of the cellular membrane in which they reside. They are activated by polyunsaturated fatty acids including arachidonic acid and by mechanical force. The 3.8-Å crystal structure of the human TRAAK channel is the first of a two-pore domain potassium ion channel. It reveals an unprecedented helical cap structure on the extracellular side of the membrane that prevents certain toxins from venomous animals from blocking its membrane environment. The structure provides a framework for future studies probing the mechanistic basis for modulation of channel activity by chemical and mechanical stimuli.
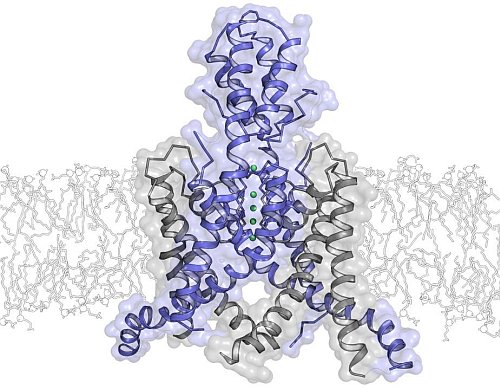 |
Figure: Ribbon representation of the TRAAK K+ ion channel viewed from the membrane plane with the extracellular milieu above. The subunits of the dimeric channel are colored blue, and gray, and K+ ions are green spheres. |
Citation:
Brohawn, SG, del Marmol, J, MacKinnon, R. Crystal structure of the human K2P
TRAAK, a lipid- and mechano-sensitive K+ ion channel, Science 335, 436-441
(2012).