 |
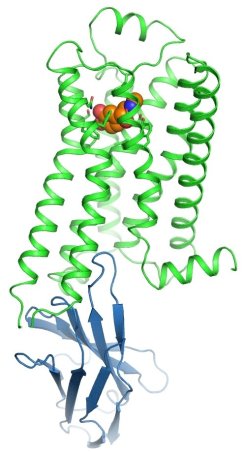
Figure: The beta 2 AR (green) bound to adrenaline (orange) and stabilized in an active state by an engineered nanobody (blue). |
The groups of Brian Kobilka, Bill Weis, and Chris Garcia at Stanford University determined the adrenaline-activated structure of the beta 2 adrenoceptor stabilized with an engineered nanobody. The beta 2 adrenergic receptor (beta 2 AR) is a G-protein-coupled receptor (GPCR) that mediates cellular responses to adrenaline. Kobilka, Weis, and collaborators have previously obtained structures of the beta 2 AR in inactive and active states, but it had not been possible to capture an active-state of a GPCR bound to its native neurotransmitter. Crystal structures of agonist-bound GPCRs have relied on the use of either exceptionally high-affinity agonists or receptor stabilization by mutagenesis. Many natural agonists such as adrenaline bind with relatively low affinity, and they are often chemically unstable. Using directed evolution, they engineered a high-affinity camelid antibody fragment (nanobody) that stabilizes the active state of the beta 2 AR, and used this to obtain crystal structures of the activated receptor bound to three chemically distinct agonists: the ultrahigh-affinity agonist BI167107, the high-affinity catecholamine agonist hydroxybenzyl isoproterenol, and the low-affinity endogenous agonist adrenaline. The crystal structures reveal a highly conserved overall ligand recognition and activation mode despite diverse ligand chemical structures and affinities that range from 100 nM to ~80 pM. Overall, the adrenaline-bound receptor structure is similar to the others, but it has substantial rearrangements in extracellular loop three and the extracellular tip of transmembrane helix 6. These structures also reveal a water-mediated hydrogen bond between two conserved tyrosines that appears to stabilize the active state of the beta 2 AR and related GPCRs.
Citation: Ring, AM, Manglik, A, Kruse, AC, Enos, MD, Weis, WI, Garcia, KC, Kobilka, BK. Adrenaline-activated structure of [beta subscript 2]-adrenoceptor stabilized by an engineered nanobody, Nature 502, 575-579 (2013). DOI: 10.1038/nature12572