The laboratory of Andrew Gulick determined structures of two nonribosomal peptide synthetase (NRPS) enzymes. The large, multidomain NRPSs use an assembly-line strategy to produce bio-active peptides including antibiotics such as vancomycin and teichobactin, anticancer agents like bleomycin, and siderophores such as mycobactin and pyoverdine, which are important virulence determinants. During synthesis, the amino-acid substrates and nascent peptide are covalently attached to peptidyl carrier protein (PCP) domains that are integrated within the multi-domain NRPS protein. Large conformational changes are therefore required for the PCP to reach the neighboring catalytic domains. The new structures illustrate the conformation of NRPS proteins in distinct states of the catalytic cycle. One structure shows the PCP domain bound within the adenylation domain where it is positioned for amino acid loading. This structure exploited a mechanism-based inhibitor conceived and synthesized by collaborator Courtney Aldrich (University of Minnesota). The second structure illustrates the PCP domain bound to the condensation domain, poised to accept the upstream peptide. The two structures demonstrate that a 140° sub-domain rotation within the adenylation domain is used to transport the PCP between the catalytic domains. The dynamics of NRPS proteins was further supported by single particle electron microscopy (EM) from Georgios Skiniotis (University of Michigan). The structures should facilitate engineering NRPS catalysts to produce novel peptide therapeutics.
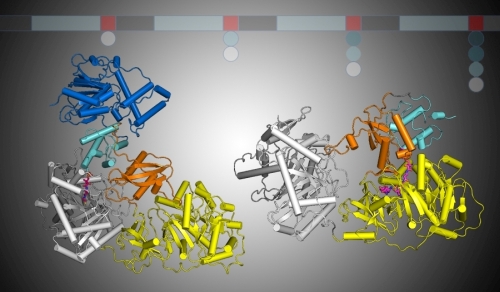 |
Figure: Two multi-domain NRPS proteins. The left panel shows the PCP (cyan) bound within the condensation domain (white). Rotation of the small C-terminal subdomain (orange) of the adenylation domain (yellow) transports the PCP to the adenylation domain active site in the structure on the right. |
Citation: Drake, E.J., Miller, B.R., Shi, C., Tarrasch, J.T., Sundlov, J.A., Allen, C.L., Skiniotis, G., Aldrich, C.C., and Gulick, A.M. (2016) Structures of two distinct conformations of holo-non-ribosomal peptide synthetases. Nature 529, 235-238.