Mark Lemmon's group of the University of Pennsylvania published a major paper aimed at defeating cancer. Members of the epidermal growth factor receptor (EGFR) family and their activating ligands are essential regulators of diverse developmental processes, and inappropriate activation of these receptors is a key feature of many human cancers. A natural secreted antagonist of EGFR signalling, called Argos, was identified in Drosophila, and the investigators previously showed that Argos functions by directly binding and sequestering growth factor ligands that activate EGFR. Using data collected at GM/CA-CAT and at the ALS, the investigators determined the crystal structure of Argos bound to an EGFR ligand, Spitz. Contrary to expectations, Argos contains no EGF-like domain. Instead, three closely-related domains, resembling a three-finger toxin fold, form a clamp-like structure around the bound EGF ligand. Although structurally unrelated to the receptor, Argos mimics EGFR by using a bipartite binding surface to entrap EGF. The individual Argos domains share unexpected structural similarities with two families of other receptors. The structures presented here define requirements for the design of artificial EGF-sequestering proteins that would be valuable anti-cancer therapeutics.
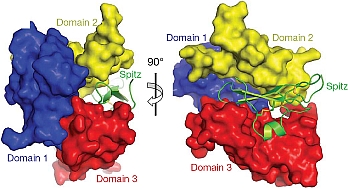 |
Figure: Argos' sequestration of Spitz. |
Citation:
Klein, DE, Stayrook, SE, Shi, F, Narayan, K, Lemmon, MA. Structural basis
for EGFR ligand sequestration by Argos, Nature 453, 1271-1275 (2008). DOI:
10.1038/nature06978.