The group of Shelagh Ferguson-Miller at Michigan State University published two papers, including a cover article in Biochemistry, on mechanistic studies of the complex and challenging membrane protein, cytochrome c oxidase (CcO). CcO couples electron and proton transport to reduction of O2 to H2O at its active site (heme a3 and CuB), meanwhile pumping protons across membranes for ATP synthesis. Structures are known for bacterial and for bovine CcOs, but questions remain about the complicated chemistry of coupled electron/proton transport, and about the binding sites for effector molecules. Two pathways in CcO take up protons; the D pathway transports protons across the membrane and also donates protons to the reduction of O2, while the K pathway only donates protons to reduction of O2. A binding site for deoxycholate was identified in the CcO K path, leading to the proposal that this is a conserved site for binding steroids and other physiological effectors (Qin, L. et al., Biochemistry, 47, 9931-9933 (2008)). In the structure of the reduced CcO (Qin, L. et al., Biochemistry, 48, 5121-5130 (2009)), redox-induced shifts in the active site and changes in water locations in the D and K paths suggest a conformational gate for cycling between proton paths in the catalytic cycle.
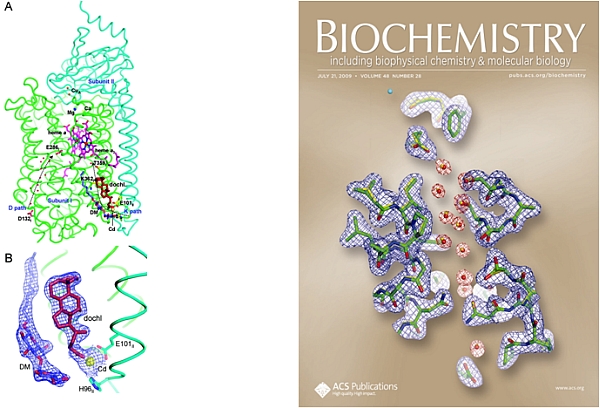 |
Figure: Channels in cytochrome c oxidase. On the left, a deoxycholate binding site and a detergent binding site were identified in the K path of R. sphaeroids CcO. On the right, comparisons of structures of the reduced and oxidized forms of R. sphaeroides CcO show the loss of a strategically located water from the D path in the reduced state. |
Citation:
| [1] | Qin, L, Mills, DA, Buhrow, L, Hiser, C, Ferguson-Miller, S, A Conserved Steroid Binding Site in Cytochrome c Oxidase, Biochemistry-US 47 (38), 9931-9933 (2008). DOI: 10.1021/bi8013483. |
| [2] | Qin, L, Liu, J, Mills, DA, Proshlyakov, DA, Hiser, C, Ferguson-Miller, S. Redox-Dependent Conformational Changes in Cytochrome c Oxidase Suggest a Gating Mechanism for Proton Uptake, Biochemistry-US 48 (23), 5121-5130 (2009). DOI: 10.1021/bi9001387. |