The group of Martin Caffrey at Trinity College in Dublin, Ireland, used the X-ray minibeam at GM/CA@APS, as well as a beamline at the Diamond Light Source in the U.K., to determine the structure of diacylglycerol kinase, which is an integral membrane enzyme involved in the synthesis of key components of the cellular envelope, including lipopolysaccharide, in Gram-negative bacteria. For 50 years, it has served as a paradigm for investigating membrane protein enzymology, folding, assembly, and stability. It is an enzyme that catalyzes a complex reaction involving substrates with widely contrasting solubilities. Its lipid substrate abhors water and resides in the fatty membrane that surrounds the cell. By contrast its second or co-substrate, ATP (which incidentally is the energy currency of the cell), is totally water soluble. How this diminutive enzyme, the smallest known kinase, manages to bring such disparate substrates together at the membrane interface for reaction is revealed in molecular detail in the new crystal structure. The protein embeds most of its bulk in the membrane where it interacts with the lipid substrate. The rest extends out of the membrane into the watery cytoplasm to sequester ATP. The two substrates are then forced into a molecular embrace at the membrane-cytoplasm interface. Residing just atomic distances apart and perfectly aligned for reaction in the maw of the enzyme, the two molecules are thought to fuse becoming a single entity, ever so briefly. Upon separating, the lipid takes with it ATP's terminal phosphate. In a series of follow up enzyme reactions, the newly formed phospholipid contributes to cell envelope synthesis. These findings are likely to attract considerable attention, in part, because they help explain almost half a century of work on this paradigmatic membrane protein. The study is notable too because the crystal structure differs profoundly from the only other structure available for this novel kinase that was obtained using solution nuclear magnetic resonance.
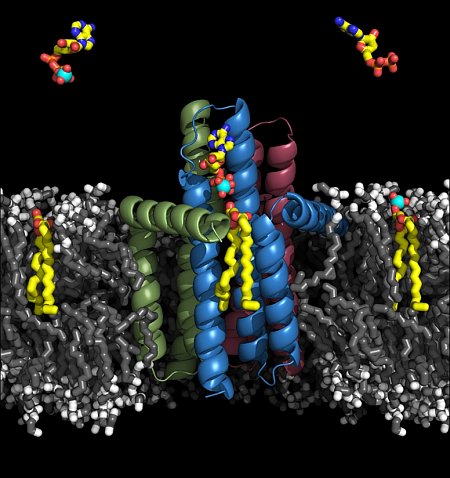 |
Figure: Rendering of the diacylglycerol kinase structure in a lipid membrane, showing substrates. |
Citation: Li, D., Lyons, A.J., Pye, V.E., Vogeley, L., Aragao, D., Kenyon, C.P., Shah, S.T.A., Doherty, C., Aherne, M., Caffrey, M. Crystal structure of the integral membrane diacylglycerol kinase. Nature 2013 May 23; 497: 521-524. DOI 10.1038/nature12179.