The Ismagilov group from the University of Chicago recently proposed to use simple guest host chemistry to modulate crystallization of membrane proteins by capturing detergents with methyl-β-cyclodextrin (MBCD) in membrane protein samples. MBCD forms a complex with detergent, breaking up free detergent micelles and extracting loosely bound detergent in the protein-detergent complex (PDC). It was shown that with detergent capture, the membrane protein sample had improved homogeneity. Using a plug-based microfluidic system, which is compatible with crystallization of membrane proteins, they screened for the optimal concentration of MBCD as additive and crystallized reaction center (RC) from Blastochloris viridis in a different space group than had been previously observed. Using the crystals grown directly in their 10-nL plugs, they solved the structure to 3.2 Å, with an X-ray diffraction experiment performed at GM/CA CAT's 23ID-D. The RC crystals in the new space group show protein contacts closer to the membrane plane. This observation supported their hypothesis that MBCD extracted detergent loosely bound to protein, thus making more residues accessible to protein-protein contacts. The Ismagilov group also proposed to add MBCD during the process of concentrating membrane proteins, in order to reduce the detergent concentration in the concentrated sample and prevent the formation of free micelles.
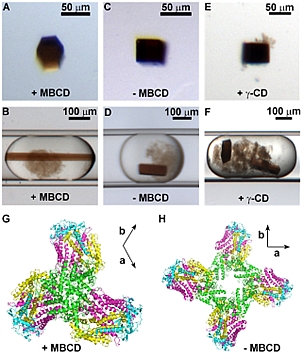 |
Figure: MBCD captures free and loosely-bound detergent and changes the morphology and packing of RC crystals. (A) Trigonal crystal grown with 4 mM MBCD and 4.6 mM LDAO. (B) Trigonal crystal in a microfluidic plug. (C) Tetragonal crystal grown at 44 mM LDAO (~ 40 CMC). (D) Tetragonal crystal in a microfluidic plug. (E) Tetragonal crystal grown with 8 mM γ-CD and 4.6 mM LDAO. (F) Tetragonal crystals in a plug containing 8 mM γ-CD. (G) Packing of RC proteins in trigonal crystals P3121, a = b = 241.2 Å, c = 113.4 Å. (H) Packing of RC proteins in tetragonal crystals P43212 a = b = 220.4 Å, c = 113.0 Å. (G) and (H) are viewed along the c axis (31 axis for G and 43 axis for H). The a and b axial directions are shown on the top right of each figure. Green: subunit C; cyan: subunit H; yellow: subunit M; and pink: subunit L. |
Citation:
Li, L, Mustafi, D, Fu, Q, Tereshko, V, Chen, DL, Tice, JD, Ismagilov, RF.
Nanoliter microfluidic hybrid method for simultaneous screening and
optimization validated with crystallization of membrane proteins, Proc. Natl.
Acad. Sci. USA 103 (51), 19243-19248 (2006). DOI: 10.1073/pnas.0607502103