Landmark structures of the human β2-adrenergic G-protein-coupled receptor were determined by the groups of Brian Kobilka and Bill Weis of Stanford University, and Ray Stevens and Peter Kuhn of the Scripps Research Institute, resulting in 3 publications in Nature and Science in late 2007, and another paper in Structure in 2008 (Rasmussen et al., Nature 450, 383-387 (2007); Cherezov et al., Science 318, 1258-1265 (2007); Rosenbaum et al., Science 318, 1266-1273 (2007); Hanson et al., Structure 16, 897-905 (2008)). This integral membrane protein which functions in cardiovascular and pulmonary physiology is activated by adrenaline, and responds to beta blockers. It is a well-studied member of the G protein-coupled receptor (GPCR) family. The GPCR family contains almost 1,000 members, who play critical roles in heart disease, blood pressure regulation, inflammation, and psychological disorders. More than half of all therapeutic drugs target one GPCR or another. Different GPCRs respond to a wide array of signals, including light, hormones, neurotransmitters, proteins, and other signals. Indeed, the only other known GPCR structure until now was that of rhodopsin, the visual receptor. This was a challenging project, as GPCRs other than rhodopsin tend to occur in low natural abundance, have inherent structural flexibility, and suffer instability in detergent solutions. Extraordinary efforts were undertaken, including massive screening of crystallization conditions with and without lipidic cubic phases, and stabilization of protein via Fab binding and by fusion-protein constructs. Some initial data were collected at the ESRF for the first paper, but GM/CA-CAT's mini-beam capabilities played a pivotal role in all of the structure determinations. This project involved a collaboration with GM/CA staff to enhance the mini-beam capabilities to improve the user friendliness and robustness of the device. The 5-micron mini-beam provided high signal-to-background data from the small crystals and allowed the investigators to overcome radiation damage by "raster collecting" data along rod-like crystals. Crystals in the lipidic cubic phase could not be seen visually prompting the development of the diffractive and fluorescence rastering techniques to locate the crystals. The results provide insight on GPCR structural plasticity and stability compared to rhodopsin, they show some modes of ligand binding, and they further understanding of signal transduction by GPCRs.
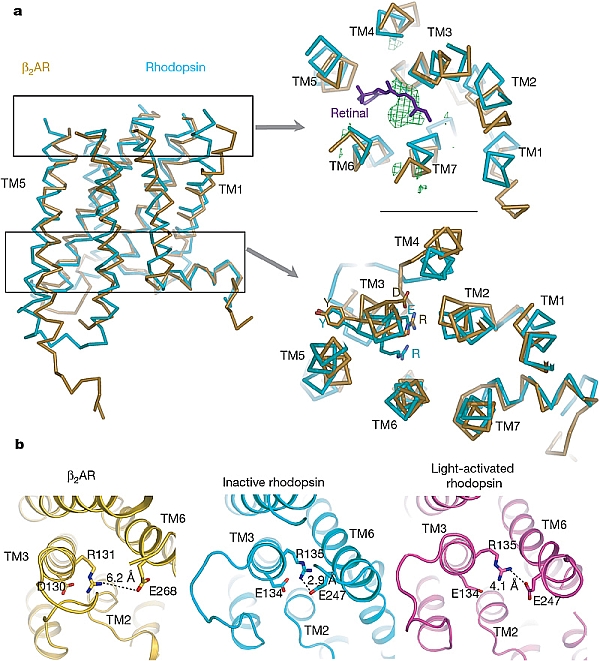 |
Figure: A comparison of the first structure of human β2-adrenergic G-protein-coupled receptor to rhodopsin, from Rasmussen et al. (full citation below) [Figure reprinted by permission from Macmillan Publishers Ltd: Nature, 2007] |
Citation:
| [1] | Rasmussen, SGF, Choi, H-J, Rosenbaum, DM, Kobilka, TS, Thian, FS, Edwards, PC, Burghammer, M, Ratnala, VRP, Sanishvili, R, Fischetti, RF, Schertler, GFX, Weis, WI, Kobilka, BK. Crystal structure of the human β2 adrenergic G-protein-coupled receptor, Nature 450, 383-387 (2007). DOI: 10.1038/nature06325. |
| [2] | Cherezov, V, Rosenbaum, DM, Hanson, MA, Rasmussen, SGF, Thian, FS, Kobilka, TS, Choi, H-J, Kuhn, P, Weis, WI, Kobilka, BK, Stevens, RC. High-Resolution Crystal Structure of an Engineered Human β2-Adrenergic G Protein-Coupled Receptor, Science 318, 1258-1265 (2007). DOI: 10.1126/science.1150577. |
| [3] | Rosenbaum, DM, Cherezov, V, Hanson, MA, Rasmussen, SGF, Thian, FS, Kobilka, TS, Choi, H-J, Yao, X-J, Weis, WI, Stevens, RC, Kobilka, BK. GPCR Engineering Yields High-Resolution Structural Insights into β2 Adrenergic Receptor Function, Science 318, 1266-1273 (2007). DOI: 10.1126/science.1150609. |
| [4] | Hanson, MA, Cherezov, V, Griffith, MT, Roth, CB, Jaakola, V-P, Chien, EYT, Velasquez, J, Kuhn, P, Stevens, RC. A Specific Cholesterol Binding Site Is Established by the 2.8 Aring; Structure of the Human β2-Adrenergic Receptor, Structure 16 (6), 897-905 (2008). DOI: 10.1016/j.str.2008.05.001. |