In difficult RNA work, Barbara Golden's group has solved the structure of a tyrosyl-tRNA synthetase splicing factor bound to a group I intron RNA. CYT-18 protein is a mitochondrial tRNA synthetase that also moonlights as a group I intron splicing factor. The structure of a complex between CYT-18 and a group I intron was determined by collecting data at GM/CA-CAT and SER-CAT. These results provide insight on how CYT-18 promotes group I intron splicing, how it evolved to have this function, and how proteins could have incrementally replaced RNA structures during the transition from an RNA world to an RNP world.
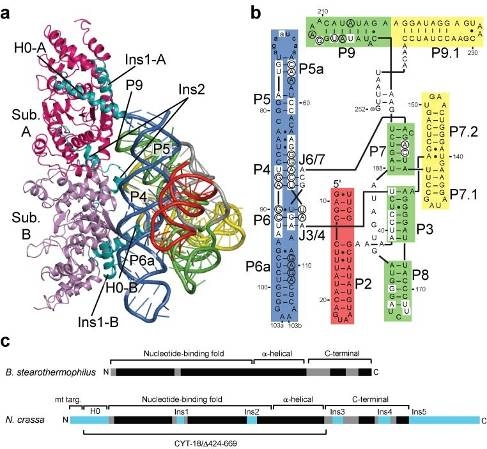 |
Figure: (A) The crystal structure of the protein-dependent RNA catalyst ('RNPzyme'). The protein (shown in magenta and pink) binds to a conserved face on the group I intron (shown in blue, red, green and yellow coils) on the face opposite that which binds tRNA (not shown). Insertions peculiar to the splicing tRNA synthetases grip the intron, stabilizing the structure of the domain- domain interfaces within the group I RNA. (B) The secondary structure of the ribozyme. (C) Alignment of a non-splicing tyrosyl-tRNA sythetase with CYT-18. [Image courtesy of Paul Paukstelis, Alan Lambowitz, and Barbara Golden.] |
Citation:
P.J. Paukstelis, J.-H. Chen, E. Chase, A.M. Lambowitz, B.L. Golden, Structure
of a tyrosyl-tRNA synthetase splicing factor bound to a group I intron RNA,
Nature 451 (7174), 94-97 (2008). DOI: 10.1038/nature06413.