Chris Miller's group of Brandeis University studies controlled movement of solutes across biological membranes, which is managed by two types of integral membrane proteins - channels, and transporters. Channels allow for passive diffusion down a thermodynamic gradient, whereas transporters operate by a cycle of conformational changes, usually coupled to energy sources to drive substrates thermodynamically uphill. Accordingly, channel-mediated movement of substrates tends to be orders of magnitude faster than that of transporters. The peculiar CLC family of proteins, which allow the movement of Cl- across membranes in most organisms, has members that are Cl- channels, and members that are Cl-/H+ antiporters. Only the structure of the antiporter has been previously determined. In this creative work, the Miller group performed a kind of "mechanistic metamorphosis" to convert a proton-coupled chloride "antiporter" protein into a chloride channel. They wanted to see if some mutational surgery could "break" the antiporter, by removing putative conformational "gates", and thus turn it into a transmembrane continuous pathway selective for Cl ion. They were successful in this endeavor, and using data obtained at GM/CA-CAT and at beamline X29 of the NSLS, they were able to determine the structure of the mutant. The artificially-designed channel is very narrow, and slow; the authors expect natural CLC channels to have a significantly larger channel.
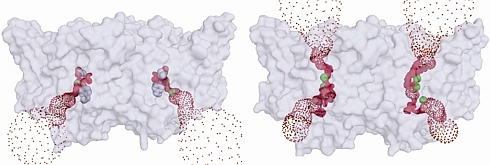 |
Figure: Images of the Cl- conduits of the antiporter (left), and the doubly-ungated mutant (right), visualized with the program HOLE. |
Citation:
Jayaram, H, Accardi, A, Wu, F, Williams, C, Miller, C. Ion permeation
through a Cl--selective channel designed from a CLC
Cl-/H+ exchanger, Proc. Natl. Acad. Sci. USA 105 (32),
11194-11199 (2008). DOI: 10.1073/pnas.0804503105.