Ming Lei's group at the University of Michigan used data collected data at GM/CA-CAT and LS-CAT to solve structures of telomere binding proteins that protect chromosomes and recruit other proteins to interact with the chromosome. Mammalian telomeres are protected by a six-protein complex, shelterin. Shelterin contains two closely related proteins, TRF1 and TRF2, which recruit various proteins to telomeres. The investigators dissected the interactions of TRF1 and TRF2 with their shared binding partner, TIN2, and other shelterin accessory factors. TRF1 recognizes a short peptide of TIN2, TIN2TBM (TIN2 TRF binding motif), using a conserved molecular surface in its TRF homology (TRFH) domain. However, this same surface does not act as a TIN2 binding site in TRF2. Instead, the TRFH docking site of TRF2 binds a shelterin accessory factor Apollo, which does not interact with the TRFH domain of TRF1. Conversely, the TRFH domain of TRF1, but not of TRF2, interacts with another shelterin associated factor PinX1. These studies reveal that TRF1 and TRF2 share a versatile protein interaction interface that functions as a protein docking site to recruit different factors to telomeres by specifically interacting with either TRF1 or TRF2.
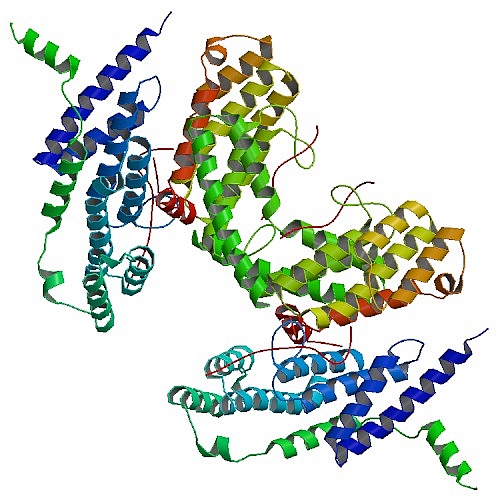 |
Figure: Crystal Structure of TRF2 TRFH domain and TIN2 peptide complex [PDB ID 3BU8] |
Citation:
Chen, Y, Yang, Y, van Overbeek, M, Donigian, JR, Baciu, P, de Lange, T, Lei,
M. A Shared Docking Motif in TRF1 and TRF2 Used for Differential Recruitment
of Telomeric Proteins, Science 22, 1092-1096 (2008).
DOI:10.1126/science.1151804.