Ronen Marmorstein's group at the Wistar Institute published an exciting advance towards anti-cancer therapy. The authors described an approach of using structure-based design to develop a novel class of potent organoruthenium inhibitors that displayed excellent selectivity in vitro to phosphatidyl-inositol-3-kinase (PI3K) over a range of other representative protein kinases. The most potent inhibitor was found to potently and specifically inhibit the PI3K signaling pathway in a melanoma cell line, and it was also found to strongly inhibit human melanoma growth and invasiveness. The crystal structure solved from the data collected at GM/CA-CAT beamline 23ID-D helped to elucidate the structural features that contribute to the high specificity of this novel class of organoruthenium inhibitors to PI3K. In addition, the structural evidence provides a potential means to design of organometallic PI3K inhibitors that possess specificity towards different PI3K isoforms.
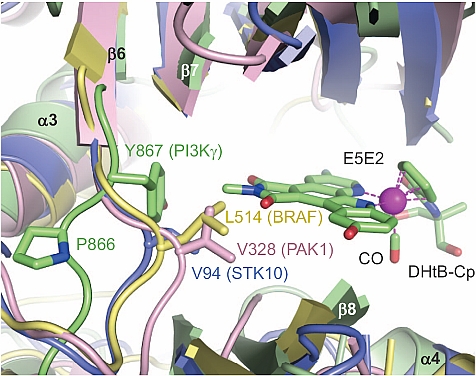 |
Figure: The structure of the E5E2 inhibitor bound to PI3K (green), is overlaid with three other protein kinases (blue, yellow, pink). |
Citation:
Xie, P, Williams, DS, Atilla-Gokcumen, GE, Milk, L, Xiao, M, Smalley, KSM,
Herlyn, M, Meggers, E, Marmorstein, R. Structure-Based Design of an
Organoruthenium Phosphatidyl-inositol-3-kinase Inhibitor Reveals a Switch
Governing Lipid Kinase Potency and Selectivity, Chem. Biol. 3 (5), 305-316
(2008). DOI: 10.1021/cb800039y