The GM/CA mini-beam and rastering capabilities are key to obtaining data from the tiny membrane-protein crystals grown in the lipidic cubic phase. Raymond Stevens' group of the Scripps Research Institute extended their previous work on G protein-coupled receptors (GPCRs) to the human A2A adenosine receptor, bound to a highly selective antagonist, ZM241385. Extracellular adenosine has several regulatory roles in the central nervous system through activation of four different GPCR subtypes. The A2A receptor subtype is involved in control of locomotive behavior in basal ganglia, and is blocked by methylxanthines, such as caffeine. A correlation of coffee consumption with reduced risk of Parkinson's disease has been linked to caffeine interaction with the A2A adenosine receptor. In order to develop therapeutics for treatment of Parkinson's disease and other adenosine-receptor-related maladies (pain, Huntington's disease, asthma, seizures), compounds selective for the A2A subtype are needed. The structure revealed a different ligand-binding mode for the A2A adenosine receptor than for other GPCRs, and is the basis for compound design.
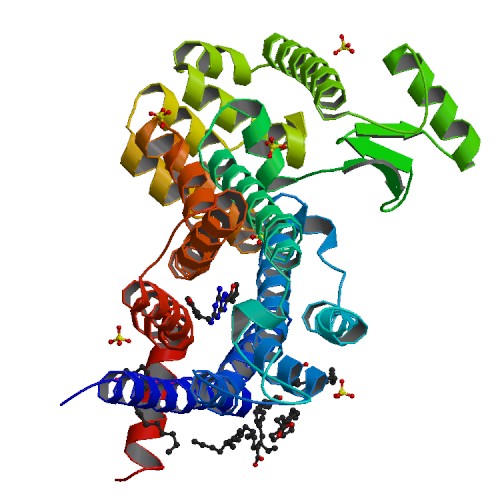 |
Figure: Crystal Structure of a Human A2A Adenosine Receptor bound to ZM241385 [PDB ID 3EML] |
Citation:
Jaakola, V-P, Griffith, MT, Hanson, MA, Cherezov, V, Chien, EYT, Lane, JR,
IJzerman, AP, Stevens, RC. The 2.6 Angstrom Crystal Structure of a Human
A[subscript 2A] Adenosine Receptor Bound to an Antagonist, Science 322,
1211-1217 (2008). DOI: 10.1126/science.1164772