Using beamline 23ID-D and small-angle X-ray studies, Mark Lemmon's group of the University of Pennsylvania has gained new insight on the orphan receptor tyrosine kinase ErbB2, which is an important therapeutic target in human cancer. ErbB2 is unique among the four human ErbB receptors, including epidermal growth factor receptor (EGFR), in its ability to transform cells in absence of ligand when it is over-expressed. Ligand-induced activation of EGFR involves conformational changes in the extracellular region, where an exposed "dimerization arm" of domain II converts from a "tethered" state to an "extended" state. Previous structures of ErbB2 were in an "extended" state, consistent with a constituitively active "auto-activated" receptor that can form dimers independent of growth factor. The new structure shows that this model is unlikely to pertain to the ErbB2 sub-family. Drosophila melanogaster EGFR (dEGFR), although a close relative of ErbB2, is strongly regulated by ligands. Like ErbB2, dEGFR also is in an "extended" state. The new structure provides several mechanistic possibilities, all different from the hEGFR model.
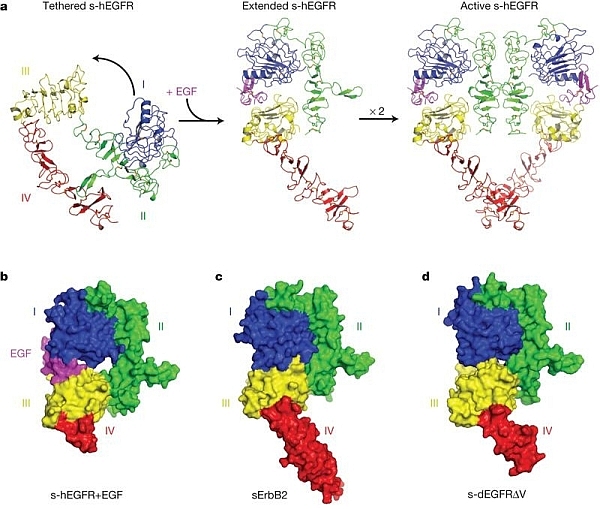 |
Figure: (A) tethered, extended, and
active states of human EGFR, with purple EGF, (B) human EGFR with bound EGF, |
Citation:
Alvarado, D, Klein, DE, Lemmon, MA. ErbB2 resembles an autoinhibited
invertebrate epidermal growth factor receptor, Nature 461, 287-291 (2009).
DOI: 10.1038/nature08297