Michael Rossmann's group at Purdue University employed a combination of X-ray crystallography and electron cryo-microscopy (cryo-EM) to investigate the mechanism of genome ejection from mature bacteriophage T4 capsids during infection. To gain insight on the process of genome ejection into the host cell, crystal structures of gp6, a baseplate protein, and gp18, a contractile tail sheath protein, were determined and fit into maps determined from electron microscopy (Aksyuk et al. Structure 17, 800-808 (2009); EMBO J. 28, 821-829 (2009)). This demonstrated that subunit motions are associated with sheath contraction and genome injection.
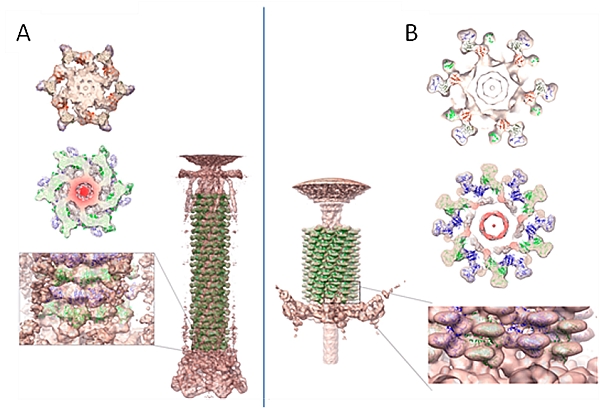 |
Figure: The role of gp18 in T4 tail
contraction. (A) The crystal structure of gp18 fit into |
Citation:
| [1] | Aksyuk, AA, Leiman, PG, Shneider, MM, Mesyanzhinov, VV, Rossmann, MG. The Structure of Gene Product 6 of Bacteriophage T4, the Hinge-Pin of the Baseplate, Structure 17 (6), 800-808 (2009). DOI: 10.1016/j.str.2009.04.005. |
| [2] | Aksyuk, AA, Leiman, PG, Kurochkina, LP, Shneider, MM, Kostyuchenko, VA, Mesyanzhinov, VV, Rossmann, MG. The tail sheath structure of bacteriophage T4: a molecular machine for infecting bacteria, EMBO J. 28 (7), 821-829 (2009). DOI: 10.1038/emboj.2009.36. |