The groups of Brian Kobilka and Bill Weis at Stanford University made new advances in very challenging membrane-protein studies of beta adrenergic receptors, which represent the largest group of targets for pharmaceuticals. These receptors mediate most cell responses to extracellular hormones and neurotransmitters, resulting in activation of G proteins that change the level of intracellular messengers, such as Ca2+, cAMP, or signaling lipids. How ligand binding to the extracellular surface leads to transmembrane signal transduction has been unclear. Structures of inhibited states (with bound inverse-agonist ligands) of beta2 adrenergic receptors were determined previously using the mini-beam and rastering capabilities at GM/CA-CAT; comparison of these to the structure of an inhibited beta1 adrenergic receptor show that their orthosteric binding pockets are nearly identical in sequence, but side chains in important extracellular loops 2 and 3 (ECL2, ECL3) are only ~50% identical in sequence. The chemical environment of a salt link between ECL3 Lys 305 and ECL2 Asp 192 was probed by NMR under different ligand-binding conditions. A crystal structure from data taken at GM/CA provided a structural foundation and important control to show that 13C labeling of Lys 305 did not alter the protein structure. The NMR studies revealed that small-molecule drugs that serve as agonists, neutral antagonists, and inverse agonists stabilize distinct conformations of the extracellular surface, and provide conformational coupling between the surface and the orthosteric binding site.
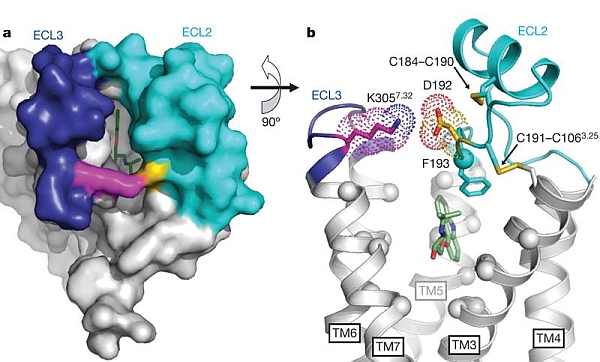 |
Figure: (A) extracellular surface of carazolol-bound (inverse agonist) beta2 adrenergic receptor, with cyan ECL2, dark blue ECL3, magenta Lys 305, yellow Asp 192, and green carazolol, (B) detailed intramolecular and ligand-binding interactions with transmembrane helices 1 and 2 omitted for clarity. [Figure reprinted by permission from Macmillan Publishers Ltd: Nature, copyright 2010] |
Citation:
Bokoch, MP, Zou, Y, Rasmussen, SGF, Liu, CW, Nygaard, R, Rosenbaum, DM, Fung,
JJ, Choi, H-J, Thian, FS, Kobilka, TS, Puglisi, JD, Weis, WI, Pardo, L,
Prosser, RS, Mueller, L, Kobilka, BK. Ligand-specific regulation of the
extracellular surface of a G-protein-coupled receptor, Nature 463, 108-112
(2010). DOI: 10.1038/nature08650