The group of Carrie Wilmot at the University of Minnesota obtained in crystallo snapshots of post-translational cofactor formation in the MauG/pre-methylamine dehydrogenase complex. Methylamine dehydrogenase (MADH), a metabolic enzyme of methylotrophic and autotrophic bacteria, catalyzes the oxidative deamination of a primary amine to aldehyde. MADH requires a rare tryptophan tryptophylquinone (TTQ) cofactor, which is formed post-translationally by covalent linkage of two tryptophans via six-electron oxidation. MauG, a diheme enzyme, completes the synthesis of the TTQ cofactor from a monohydroxylated tryptophan and an unmodified tryptophan in the MADH precursor known as preMADH. The 2.1-Å structure of the 203.6-kDa MauG/preMADH complex revealed a long 40.1-Å separation between the nascent TTQ site and the most distant heme of MauG. The proximal (low spin) MauG heme had an unusual six-coordinate heme iron with His and Tyr axial ligands; the distal (high spin) heme, which is thought to form an Fe(IV)=O (ferryl) intermediate during catalysis, was five-coordinate. The authors treated crystals with excess hydrogen peroxide and observed formation of TTQ in the crystal, as well as extra electron density contiguous with the distal heme iron. This remarkable post-translational biosynthetic reaction is both substrate-assisted and employs long-range catalysis.
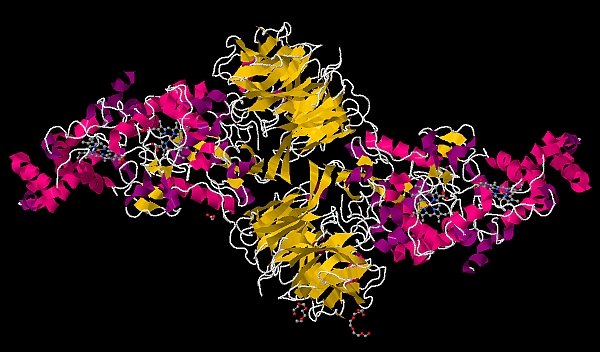 |
Figure: MauG/preMADH complex after treatment of crystals with H2O2 [PDB ID 3L4O] |
Citation:
Jensen, LMR, Sanishvili, R, Davidson, VL, Wilmot, CM. In Crystallo
Posttranslational Modification Within a MauG/Pre-Methylamine Dehydrogenase
Complex, Science 327, 1392-1394 (2010). DOI: 10.1126/science.1182492