The group of Krzysztof Palczewski at Case Western Reserve University made major insights into the mechanism of bovine RPE65, a key enzyme in the visual cycle. Data from GM/CA 23ID-D and from the NSLS were used in the 2.14-Å structure determination (Kiser et al., Proc. Natl. Acad. Sci. USA 106 17325-17330 (2009)), and from 23ID-D in work at 1.9-Å (Golczak et al., J. Biol. Chem. 285 9667-9682 (2010)). The visual cycle is initiated when the 11-cis-retinylidene chromophore of rhodopsin is photo-isomerized to all-trans-retinylidene. All-trans-retinylidene is re-cycled through the retinal pigment epithelium (RPE) as an all-trans-retinyl ester of phosphatidyl choline. RPE65 catalyzes ester hydrolysis and the key isomerization from trans to cis before re-incorporation into rhodopsin. Mutations in RPE65 cause a hereditary childhood blinding disease called Leber congenital amaurosis, and also retinitis pigmentosa, the degenerative blinding disease that affects 2 million people worldwide. RPE65 has a seven-bladed beta-propeller in which one residue from each propeller is in the first (4 His) or second (3 Glu) coordination shell of the catalytic iron. Like other enzymes with substrates in the membrane bilayer, a hydrophobic tunnel leads to the protein surface at an amphipathic helix. Most of the disease-related mutations are at residues that maintain the structure of the active site. The structures also laid to rest a high-profile hypothesis that membrane association and activity are regulated by reversible S-palmitoylation at certain Cys residues, which are neither surface exposed nor palmitoylated.
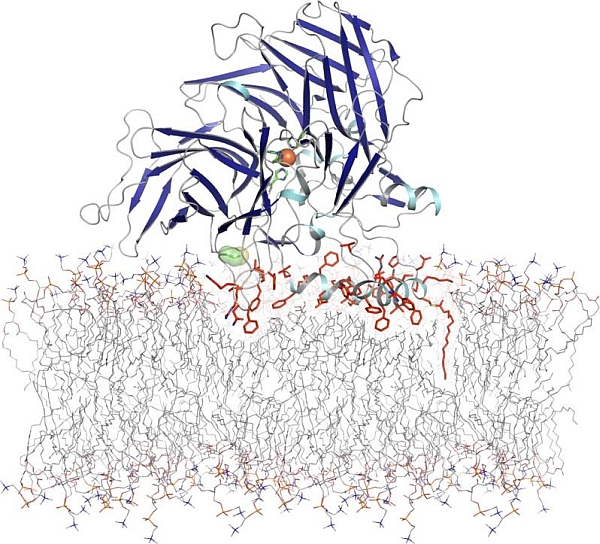 |
Figure: RPE65 topology relative to a phosphatidylcholine-containing bilayer. [This figure was originally published in the Journal of Biological Chemistry (full citation below), copyright of the American Society for Biochemistry and Molecular Biology."] |
Citation:
| [1] | Kiser, PD, Golczak, M, Lodowski, DT, Chance, MR, Palczewski, K. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle, Proc. Natl. Acad. Sci. USA 106, 17325-17330 (2009). DOI: 10.1073/pnas.0906600106. |
| [2] | Golczak, M, Kiser, PD, Lodowski, DT, Maeda, A, Palczewski, K. Importance of Membrane Structural Integrity for RPE65 Retinoid Isomerization Activity, J. Biol. Chem. 285, 9667-9682 (2010). DOI: 10.1074/jbc.M109.063941. |