The Kobilka and Weis groups and collaborators made a major step forward in the study of transmembrane signal transduction. G protein coupled receptors (GPCRs) exhibit a spectrum of functional behaviors in response to natural and synthetic ligands. Recent crystal structures provide insights into inactive states of several GPCRs. Efforts to obtain an agonist-bound active-state GPCR structure have proven difficult due to the inherent instability of this state in the absence of a G protein. The authors generated a camelid antibody fragment (nanobody Nb80) to the human β2 adrenergic receptor (β2AR) that exhibits G protein-like behavior, and obtained an agonist-bound, active-state crystal structure of the receptor-nanobody complex. Comparison with the inactive β2AR structure reveals subtle changes in the binding pocket; however, these small changes are associated with an 11 Å outward movement of the cytoplasmic end of transmembrane segment 6, and rearrangements of transmembrane segments 5 and 7 that are remarkably similar to those observed in opsin, an active form of rhodopsin. This structure provides insights into the process of agonist binding and activation.
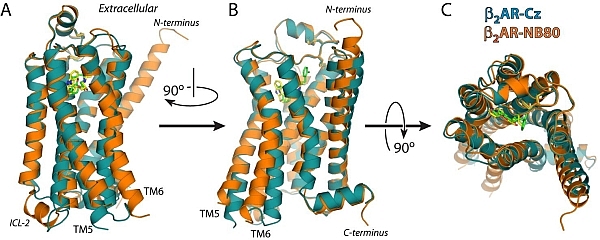 |
Figure: Comparison of the inverse-agonist and agonist-Nb80-stabilized crystal structures of the β2AR. The structure of inverse agonist carazolol-bound β2AR-T4L (β2AR-Cz) is shown in blue with the carazolol in yellow. The structure of BI-167107 agonist-bound and Nb80-stabilized β2AR-T4L (β2ARNb80) is shown in orange with BI-167107 in green. (A): side view of the superimposed structures showing significant structural changes in the intracellular and G protein facing part of the receptors, (B): side view following 90° rotation on the vertical axis, and (C): comparison of the extracellular ligand binding domains showing modest structural changes. |
Citation:
Rasmussen, SGF, Choi, H-J, Fung, JJ, Pardon, E, Casarosa, P, Chae, PS,
DeVree, BT, Rosenbaum, DM, Thian, FS, Kobilka, TS, Schnapp, A, Konetzki, I,
Sunahara, RK, Gellman, SH, Pautsch, A, Steyaert, J, Weis, WI, Kobilka, BK.
Structure of a nanobody-stabilized active state of the β2
adrenoceptor, Nature 469, 175-180 (2011). DOI: 10.1038/nature09648