George Phillips' group at the University of Wisconsin - Madison advanced PSI efforts to understand the synthesis of microbial natural products. Microbial natural products are interesting for many reasons, including their potential use as drugs. The compound calicheamicin comprises an enediyne, which acts as a "warhead" to cleave the double-stranded DNA of other microbes. The warhead is decorated with various modified carbohydrates, which affect the solubility and transport of the molecule. Structures were solved for all four glycosyltransferase enzymes that attach the sugars to the warhead . The proteins, known as CalG1, CalG2, CalG3 and CalG4, share a common mechanism for positioning the sugars, but orient the acceptor molecule differently through differences in their N-terminal domains.
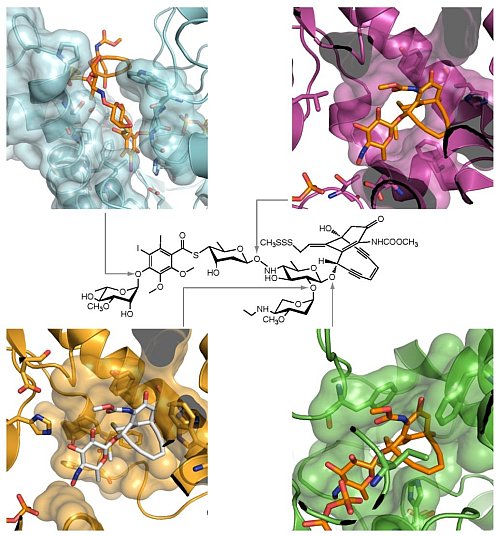 |
Figure: Glycosyltransferases of calicheamicin biosynthesis. Calicheamicin is shown at the center, surrounded by the four glycosyltransferases CalG1 (light blue), CalG2 (purple), CalG3 (green) and CalG4 (gold). |
Citation:
Chang, A, Singh, S, Helmich, KE, Goff, RD, Bingman,
CA, Thorson, JS, Phillips, GN, Jr. Complete set of glycosyltransferase
structures in the calicheamicin biosynthetic pathway reveals the origin of
regiospecificity, Proc Natl Acad Sci U S A 108, 17649-17654 (2011).