With more than 2000 members identified, ATP-binding cassette (ABC) transporters are transmembrane proteins that constitute one of the largest protein superfamilies. They are integral to most biological processes and many are medically important. ABC transporters function through the alternating-access model, which is the most general mechanism of membrane transport. Despite decades of study and recent rapid progress, two fundamental questions remain unanswered: (1) Do intermediate conformations exist between the inward- and outward-facing states? (2) How does the presence of substrate initiate the transport cycle? The Chen group addressed both of these questions using the maltose transport system of E. coli, which has long been the prototype for studies of ABC transporters; they determined the crystal structure of an essential intermediate, the pretranslocation state, which exists between the inward- and outward-facing states in the alternating access model. This structure shows that interactions with the substrate-binding protein prime the transporter for ATP-induced conformational change by influencing the conformation of the nucleotide-binding domains on the other side of the membrane.
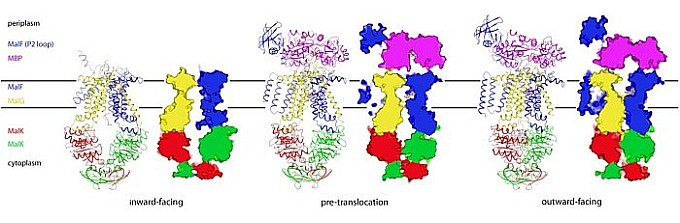 |
Figure: Membrane translocation states of the maltose transporter – wire representations, and solid representations: MalF (blue), MBP (lilac), MalG (yellow), MalK (red, green). |
Citation: Oldham ML, Chen J. Crystal Structure of the Maltose Transporter in a Pretranslocation Intermediate State. Science. 2011 June 3; 332; 1202-5. doi: 10.1126/science.1200767.