Protein kinase C (PKC) isozymes are the paradigmatic effectors of lipid signaling. PKCs regulate a remarkable range of physiological pathways, including, but not limited to, T-cell recognition, cell polarity, cell migration, proliferation and differentiation, neuronal signaling and metabolism. PKC isozymes are regulated by a series of phosphorylation events critical to their enzymatic activity and structural integrity. Despite a large body of fragmentary structural information for the isolated domains, a full mechanistic understanding has proved elusive, in part due to challenges in crystallizing a full-length isoform of PKC. James Hurley’s group determined the 4.0-Å crystal structure of full-length Protein Kinase C βII in a partially open conformation. Diffraction data were obtained from micro-crystals measuring just 5 µm x 10 µm using the micro-beam capabilities of beamline ID23. Analysis of the structure identified an unexpected mechanism of allosteric regulation through plasticity of the C-terminal tail of the kinase domain. The C1B domain clamps a novel helix in the tail in a position that sequesters the ATP-binding Phe629 of the conserved NFD motif in a catalytically unproductive conformation. The investigators were able to confirm the crystallographic inference by mutational analysis of PKCbII membrane translocation in vivo. Thus, the structure provides a snapshot of an intermediate in the lipid activation pathway of PKCβII. PKCβII is allosterically regulated by clamping of the NFD helix by the C1B domain; the clamp is reversed upon membrane binding, defining a new structural class of protein kinase regulation.
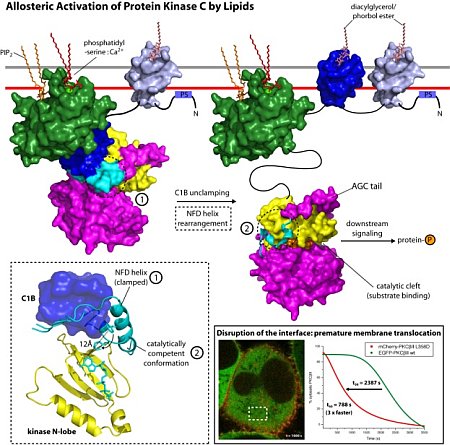 |
Figure: Left: model for a multi-step activation of PKCβII by lipids, where C1B is shown in blue, and the NFD motif is shown in cyan; Middle: close up of C1B, NFD in clamped and in active conformations, and the kinase N-lobe; Right: Mutants disrupting the C1B-kinase interface result in premature membrane translocation. |
Citation: Leonard TA, Różycki, Saidi LF, Hummer G, Hurley JH. Crystal Structure and Allosteric Activation of Protein Kinase C βII. Cell. 2011 Jan 7; 144 (1); 55-66. doi: 10.1016/j.cell 2010.12.013.