Martin Caffrey's group at Trinity College Dublin is advancing techniques in the extremely challenging field of in meso crystallization of membrane proteins. The in meso method for crystallizing membrane proteins has had significant high profile successes, including a GPCR-Gs protein complex and a spate of GPCRs and other important membrane proteins. The method is based upon the use of bicontinuous lipidic mesophases that include the cubic and sponge phases. In further development of the method, the Caffrey group explored its applicability to extremely small proteins, i.e. those with a single transmembrane domain. They examined the utility of the method with linear gramicidin, a penta-decapeptide antibiotic "mini-protein". It worked remarkably well, providing a 1.08-Å structure for the intertwined conformation of the antibiotic. Regardless of the chain length of the lipid used to create the hosting mesophases, linear gramacidin grew crystals in an intertwined conformation. The physiological relevance of the intertwined form has been questioned, and several mechanistic proposals for how this in meso crystallized form might be important have been presented, as opposed to the head-to-head conformation. The result obtained with gramicidin highlights the utility of the in meso method with proteins having single transmembrane domains, which abound in Nature. The work sets the stage for use of the in meso method in determining high-resolution structures and insights into the function of peptides and proteins referred to as single and double 'spanners' that are integral to human health.
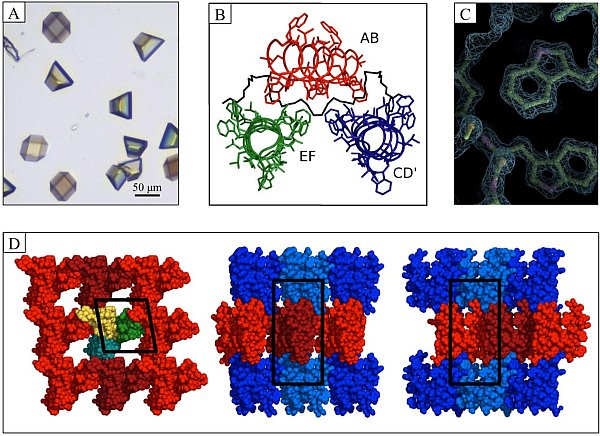 |
Figure: Linear gramicidin in meso crystals. (A) Crystals growing in meso using lipid 7.7 MAG in 28% (w/w) PEG 2000 MME, 0.1 M Bis-Tris pH 6.5. (B) Asymmetric unit with three dimers of gramicidin in the DSDH conformation labelled as dimer AB (red), EF (green) and CD' (blue) with PEG-A in black. (C) Molecular model and 1.08-Å electron density for tryptophan residues. The quality of the diffraction data is reflected in the match between model and density. (D) Layered (Type I) lattice packing in gramicidin crystals. Individual dimers are colored yellow, green, and cyan (left panel). Alternate layers are colored red (light and dark) and blue (light and dark) to highlight Type I packing (center and right-hand panels). The unit cell is boxed in black. PDB ID: 2Y5M. |
Citation:
Hoefer, N, Aragao, D, Lyons, JA, Caffrey, M. Membrane Protein
Crystallization in Lipidic Mesophases. Hosting Lipid Effects on the
Crystallization and Structure of a Transmembrane Peptide, Cryst. Growth
Des. 11, 1182-1192 (2011). DOI: 10.1021/cg101384p