The M1 strain of the widespread gram-positive pathogen Streptococcus pyogenes (group A Streptococcus, GAS) is a prevalent cause of streptococcal toxic shock syndrome (STSS). The M1 protein expressed by this strain is sufficient in animal models of disease to recapitulate the symptoms of STSS, such as vascular leakage and severe tissue injury. This damage is brought about by integrin-dependent activation of neutrophils and other immune cells by a complex formed between the M1 protein and host fibrinogen. To investigate how complex formation brings about a massive inflammatory response, the groups of Partho Ghosh and collaborators determined the structure of an M1-fibrinogen complex, which revealed that M1 organizes four molecules of fibrinogen into a specific cross-like pattern. They found this pattern to be the fundamental unit for the formation of a supramolecular M1-fibrinogen network. This network was required for neutrophil activation and alterations to it were not tolerated. Although the M1-fibrinogen network is distinct from a fibrin clot, these supramolecular assemblies both present high densities of integrin-binding sites, indicating that integrin clustering and avidity are conserved mechanisms for leukocyte activation. Interference with the M1-fibrinogen interaction represents a potential therapeutic target to ameliorate the severe outcomes of STSS.
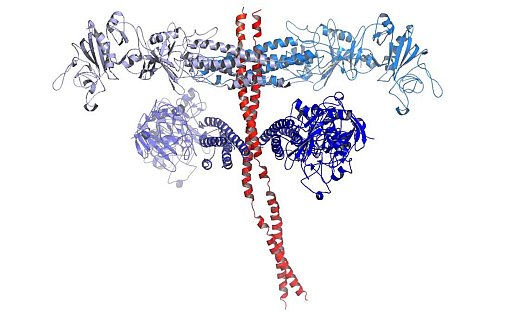 |
Figure: The M1-fibrinogen structure in ribbon representation. M1 is in red, and the four FgD molecules bound by M1 are in shades of blue. |
Citation: Macheboeuf P, Buffalo C, Fu C-y, Zinkernagel AS, Cole JN, Johnson JE, Nizet V, Ghosh P. Streptococcal M1 protein constructs a pathological host fibrinogen network. Nature. 2011 April 7; 472: 64-68. doi: 10.1038/nature09967