 |
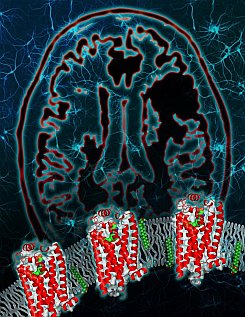
Figure: Crystal structure of the human lipid GPCR. The S1P1 receptor reveals a highly organized N-terminus that limits access of the lysophospholipid ligand sphingosine-1-phosphate to the binding pocket from the extracellular environment. The zwitterionic ligand (shown in green, red and blue) is postulated to insert in the plasma membrane and then diffuse into the receptor pocket through a tunnel deep within the lipid bilayer that is bounded by transmembrane domains I and VII. The background shows the brain and neurons, a key target for S1P1 agonists in the treatment of multiple sclerosis. (courtesy of TSRI). |
The Stevens group solved the structure of a G protein-coupled receptor (GPCR) involved in the treatment of multiple sclerosis. Lyso-phospholipid sphingosine-1-phosphate modulates lymphocyte trafficking, endothelial development and integrity, heart rate, and vascular tone and maturation by activating the sphingosine-1-phosphate receptor 1 (S1P1), a receptor belonging to a subclass of the GPCR family originally termed the endothelial differentiation gene (EDG) family of lipid receptors. Activation of the S1P1 receptor through exogenous ligands, both physiological and pharmacological, results in significant inhibition of lymphocyte re-circulation. Modulation of S1P1 is the basis for the development of the nonselective S1P receptor agonist prodrug fingolimod (GILENYA(R)), approved in 2010 for the clinical treatment of relapsing-remitting multiple sclerosis. The structure of S1P1 was determined through a collaboration of scientists from Receptos and the PSI-sponsored GPCR Network project. The S1P1 structure shows that extracellular access to the binding pocket is occluded by the amino terminus and extracellular loops of the receptor. Unlike all other human GPCR structures solved to date, ligands gain access by entering laterally between helices I and VII within the transmembrane region of the receptor. The structure, along with mutagenesis, agonist structure-activity relationship data, and modeling, provides a detailed view of the molecular recognition and requirement for hydrophobic volume that activates S1P1, resulting in the modulation of immune and stromal cell responses.
Citation:
Hanson, MA, Roth, CB, Jo, E, Griffith, MT, Scott, FL, Reinhart, G, Desale, H,
Clemons, B, Cahalan, SM, Schuerer, SC, Sanna, MG, Han, GW, Kuhn, P, Rosen, H,
and Stevens, RC. Crystal structure of a lipid G protein-coupled receptor,
Science 335, 851-855 (2012)