 |
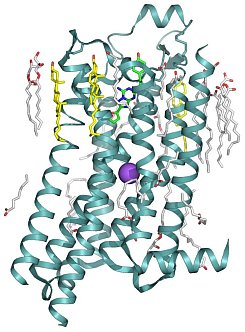
Figure: High-resolution structure of the A2A Adenosine Receptor, where sodium is shown in violet. |
Ray Stevens' group determined a high-resolution structure of the A2A adenosine receptor, which revealed the structural basis for allosteric modulation of GPCRs by sodium ions. During the past 40 years, allosteric modulation by Na+ has been observed for many GPCRs and was linked to motifs in helix II, including the highly conserved Asp2.50. Mutation of this residue has been the subject of many studies on a multitude of receptors, which have shown that the Na+ effect was largely abrogated when the residue was mutated to alanine or asparagine. Despite this indirect evidence, the nature of Na+ interactions with GPCRs remained hypothetical, and the sodium ion remained undetected in crystal structures of GPCRs solved at medium resolution. The researchers reengineered the human A2A adenosine receptor (A2AAR) by replacing its third intracellular loop with apocytochrome b562RIL and solved the structure to 1.8-Å resolution. This unprecedented high-resolution GPCR structure allowed them to identify 57 ordered water molecules inside the receptor comprising three major clusters. The central cluster harbors a putative sodium ion bound to the highly conserved aspartate residue Asp2.50. Additionally, two cholesterols stabilize the conformation of helix VI, and one of 23 ordered lipids intercalates inside the ligand-binding pocket. These high-resolution details shed light on the potential role of structured water molecules, sodium ions, and lipids/cholesterol in GPCR stabilization and function. Furthermore, this antagonist-bound structure shows that the Na+ ion is part of a water channel that spans the whole receptor; comparison with the active state structure shows dramatic rearrangement in the Na+ binding pocket.
Citation:
Liu, W, Chun, E, Thompson, AA, Chubukov, P, Xu, F, Katritch, V, Han, GW,
Roth, CB, Heitman, LH, AP, IJ, Cherezov, V, Stevens, RC. Structural basis for
allosteric regulation of GPCRs by sodium ions, Science 337, 232-236 (2012).