 |
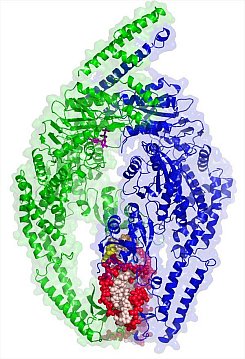
Figure: Crystal structure of MutS(beta) bound to DNA. |
Wei Yang's group at the NIH advanced research in the fight against cancer by providing key insights on mismatch recognition by MutS(beta). Mismatched base pairs resulting from misincorporation during replication lead to mutations and genomic instability if not repaired. In humans, inactivation of the MutS or MutL proteins, essential for mismatch repair, causes a condition known as Lynch Syndrome that leads to a predisposition for hereditary colorectal and other cancers. MutS(alpha), one of two MutS heterodimers in eukaryotes, is highly similar to bacterial MutS and recognizes a base mispair or 1-2 unpaired bases. In contrast, MutS(beta), a heterodimer of Msh2 and Msh3, recognizes insertion-deletion loops (IDL) of 2-15 nucleotides, as well as DNA with a 3' single-stranded overhang. Crystals of human MutS(beta) were fragile and highly radiation sensitive. The Yang group determined four structures of MutS(beta) bound to different IDL loops. MutS(beta) bends the substrate DNA much more than does MutS(alpha) and displaces the unpaired bases into the major groove rather than the minor groove as does MutS(alpha). Furthermore, the structures of MutS(beta) reveal the asymmetry between its two homologous subunits and suggest a mechanism for asymmetric modulation of ATP binding and hydrolysis by a mismatched DNA substrate. Thus association of MutS(beta) with a mismatch DNA can recruit other repair factors and signal for repair initiation.
Citation:
Gupta, S, Gellert, M, Yang, W. Mechanism of mismatch recognition revealed by
human MutS(beta) bound to unpaired DNA loops, Nat Struct Mol Biol 19, 72-78
(2012).