Youxing Jiang's group from UT-Southwestern provided a highlight on Na+/Ca2+ exchangers (NCX), which are membrane transporters that maintain the homeostasis of cytosolic Ca2+ and play an essential role in Ca2+ signaling. Despite a long history of physiological work and a large body of functional data, the structural basis underlying the ion exchange mechanism of NCX is poorly understood, and the exchanger field long sought an atomic description of an NCX protein. In this study, the Jiang group presented the first high-resolution (1.9 Å) crystal structure of a Na+/Ca2+ exchanger from Methanococcus jannaschii, NCX_Mj, and demonstrated that this archaeal NCX catalyzes Na+/Ca2+ exchange reactions similar to its eukaryotic counterpart. The high-resolution structure reveals much about the mechanism of Na+/Ca2+ exchange in NCX proteins and also explains the stoichiometry, cooperativity and bi-directionality of the ion exchange reaction in NCX.
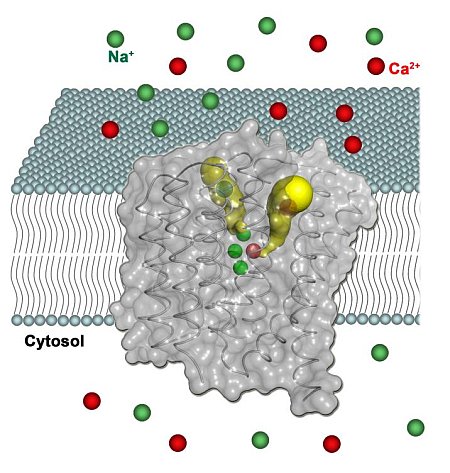 |
Figure: Crystal structure of the Na+/Ca2+ exchanger embedded in a membrane bilayer. |
Citation:
Liao, J, Li, H, Zeng, W, Sauer, DB, Belmares, R, Jiang, Y. Structural
insight into the ion-exchange mechanism of the sodium/calcium exchanger,
Science 335, 686-690 (2012).