 |
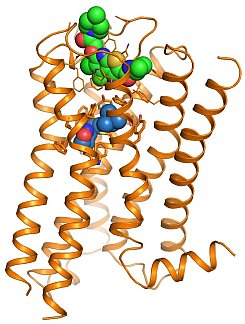
Figure: M2 muscarinic receptor (orange) bound to the agonist iperoxo (blue) and the positive allosteric modulator LY2119620 (green). |
The groups of Brian Kobilka, Bill Weis, and Chris Garcia at Stanford University investigated both activation and allosteric modulation of a muscarinic acetylcholine receptor. Muscarinic acetylcholine receptors (M1 - M5) are GPCRs that regulate the activity of a diverse array of central and peripheral functions in the human body, including the parasympathetic actions of acetylcholine. The M2 muscarinic receptor subtype plays a key role in modulating cardiac function and many important central processes such as cognition and pain perception. As it was among the first GPCRs to be cloned and purified, the M2 receptor has long served as a model system in GPCR biology and pharmacology. Muscarinic receptors have attracted particular interest due to their ability to bind small molecule allosteric modulators. Since allosteric sites are often less conserved than the orthosteric binding site, some ligands binding to allosteric sites show substantial subtype selectivity. To better understand how allosteric ligands regulate GPCR function, the group solved structures of an active-state human M2 muscarinic acetylcholine receptor bound to an agonist alone and bound to an agonist together with a positive allosteric modulator. The active state was stabilized by a G-protein mimetic antibody fragment. In addition to expected changes in the intracellular surface, the structure of the agonist-bound M2 receptor reveals larger conformational changes in the extracellular surface and the orthosteric binding site than observed in the active states of the beta 2 adrenergic receptor and rhodopsin. The structure of the M2 receptor ternary complex with an orthosteric agonist and a positive allosteric modulator reveals that the modulator recognizes a largely pre-formed binding site in the extracellular vestibule of the agonist-bound receptor. These structures offer important insights into activation mechanism and allosteric modulation of muscarinic receptors.
Citation: Kruse, AC, Ring, AM, Manglik, A, Hu, J, Hu, K, Eitel, K, H?bner, H, Pardon, E, Valant, C, Sexton, PM, Christopoulos, A, Felder, CC, Gmeiner, P, Steyaert, J, Weis, WI, Garcia, KC, Wess, J, Kobilka , BK. Activation and allosteric modulation of a muscarinic acetylcholine receptor, Nature 504, 101-106 (2013). DOI: 10.1038/nature12735