Signal transduction cascades exist in many biological systems. Jue Chen's group at Purdue University determined the structure of a maltose transporter in complex with regulatory proteins. As first documented by Jacques Monod in the 1940s, when multiple carbon sources are available, bacterial cells consume preferred carbohydrates presumably for rapid metabolism. The presence of a preferred carbon source inhibits the transport and metabolism of others, a phenomenon called carbon catabolite repression (CCR). In enteric bacteria, the central regulator of this process is the glucose specific enzyme IIA (EIIAGlc) of the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS). The influx of PTS sugars increases the level of unphosphorylated EIIAGlc, which binds and inhibits several non-PTS sugar transport systems, including the maltose transporter (MalFGK2), lactose permease, melibiose permease and raffinose permease. These carbohydrates or their derivatives function as inducers to control the synthesis of the corresponding transporters and metabolic enzymes. Thus, interaction of EIIAGlc with the transporter not only directly prevents secondary carbon source uptake, but also down regulates the expression of the corresponding catabolic systems by reducing the intracellular level of the inducer. The crystal structure of the EIIAGlc-MalFGK2 complex is the first atomic description of carbohydrate transporter regulation by CCR. Because the conformational changes that enable maltose transport have been well established through the Chen group's previous work, this structure provides a clear answer to how EIIAGlc allosterically inhibits maltose uptake in E. coli. Two EIIAGlc molecules bind symmetrically to the pivot points around which the transporter nucleotide-binding domains (NBDs) would normally rotate to form the closed dimer. By locking the NBDs in an open configuration, EIIAGlc prevents the conformational changes necessary for ATP hydrolysis and maltose transport. This work is a rare example where the structure of a membrane protein in complex with a regulatory protein has been determined by X-ray crystallography.
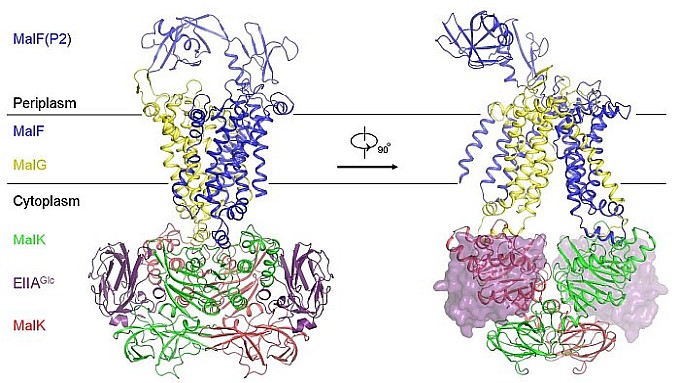 |
Figure: Crystal structure of the EIIAGlc-MalFGK2 complex. |
Citation: Chen, S, Oldham, ML, Davidson, AL, Chen, J. Carbon catabolite repression of the maltose transporter revealed by X-ray crystallography, Nature 499, 364-368 (2013). DOI: 10.1038/nature12232