 |
Figure:
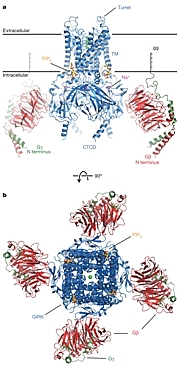
Overall structure of the GIRK- Gβγ complex. |
The binding of an agonist, such as adrenaline, acetylcholine, or glutamate, to a GPCR promotes the exchange of GDP for GTP on a bound G protein, leading to the separate dissociation of the Gα and Gβγ subunits from the receptor. The dissociated subunits then initiate the signal transduction cascade by interacting with downstream partners. Rod MacKinnon's group at the Rockefeller University determined the structure of a G-protein-gated inward rectifier K+ (GIRK) channel in complex with regulatory Gβγ subunits. GIRK channels mediate cellular electrical excitability via neurotransmitter control. In cardiac membranes, acetylcholine binding to the muscarinic acetylcholine receptor initiates this cascade. Gβγ activates GIRK channels, causing them to open, which drives the membrane voltage towards the resting potential and slows membrane depolarization. In cardiac atrial pacemaker cells, this slows the heart rate. The structure revealed one Gβγ protomer bound to each subunit of the tetrameric ion channel, which was in a pre-open state consistent with 'membrane delimited' activation of GIRK channels by G proteins and the characteristic burst kinetics of channel gating. The structure also revealed GIRK sites for the regulatory ligands phosphatidylinositol-4,5-bisphosphate (PIP2) and Na+.
Citation:
Whorton, MR, MacKinnon, R. X-ray structure of the mammalian GIRK2-[beta
gamma] G-protein complex, Nature 498, 190-197 (2013). DOI:
10.1038/nature12241