 |
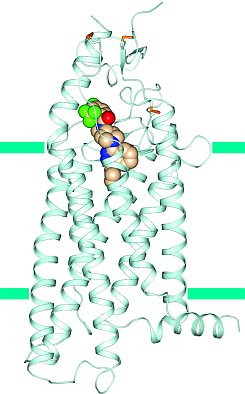
Figure: Structure of smoothened receptor, showing bound anti-tumor agent LY2940680. |
Ray Stevens' group published a G protein-coupled (GPCR) structure directly related to cancer. Smoothened receptor is a key signal transducer of the hedgehog signaling pathway, which is involved in maintenance of normal development in animals. However, abnormal hedgehog signaling is the cause for many types of cancers, and inhibition of the smoothened receptor is an effective strategy to shut down abnormal hedgehog signaling in cancerous conditions. The structure of human smoothened receptor bound to an anti-tumor agent LY2940680 was solved to a resolution of 2.5 Å using the minibeam at GM/CA@APS. The structure reveals that the smoothened receptor antagonist binds in a cavity embedded within the 7TM helical bundle. The detailed structure of this ligand binding site could provide a framework for structure based design of anticancer drug targeting the smoothened receptor. In addition, the solved structure of human smoothened receptor is the first non-class A GPCR structure reported to date. The smoothened receptor belongs to class F GPCRs, which share less than 10% sequence similarity with class A GPCRs (also known as rhodopsin-like GPCRs). Surprisingly, the transmembrane portion of the smoothened receptor is highly similar to class A GPCRs, although many motifs conserved in class A GPCRs are missing, highlighting the incredible use of 7TM fold for divergent functions.
Citation:
Wang, C, Wu, H, Katritch, V, Han, GW, Huang, XP, Liu, W, Siu, FY, Roth, BL,
Cherezov, V, Stevens, RC. Structure of the human smoothened receptor bound to
an antitumour agent, Nature 497, 338-343 (2013).