Phosphate is crucial for structural and metabolic needs including nucleotide and lipid synthesis, signaling, and chemical energy storage. Phosphate-uptake is mediated by proton-coupled transporters of the Major Facilitator Super-family in plants and fungi. A collaborative research group headed by Robert Stroud succeeded in obtaining the first high-resolution structure of a phosphate transporter using GM/CA@APS beamlines. The 2.9-Å structure of the fungal high-affinity phosphate importer was solved in an inward-facing occluded state with bound phosphate visible in the membrane-buried binding site. The structure indicated both proton and phosphate exit pathways and suggested a modified asymmetrical 'Rocker-Switch' mechanism of phosphate transport, deviating from the canonical major facilitator transport model. The phosphate transporter is related to several human transporter families, most notably the organic cation and anion transporters of the Solute Carrier Family (SLC22), which are implicated in cancer-drug resistance. The structure demonstrates and expands on principles of substrate transport by the Major Facilitator Super-family and illuminates principles of phosphate uptake in particular.
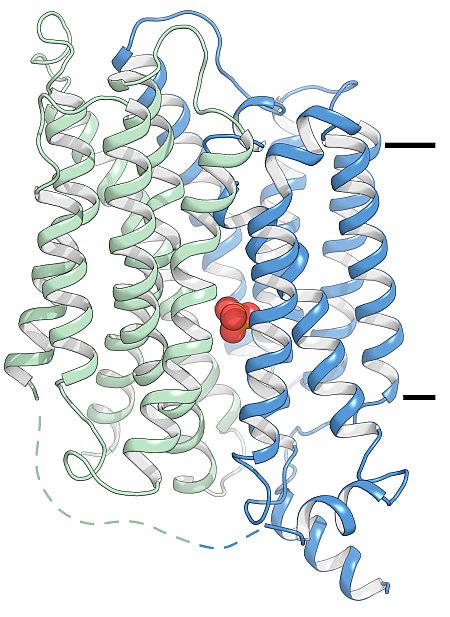 |
Figure: The high affinity phosphate transporter in ribbon representation. The two domains of the transporter are colored in blue and green and the centrally located phosphate is colored orange. The black bars denote the approximate location of the membrane. |
Citation: Pedersen BP, Kumar H, Waight AB, Risenmay AJ, Roe-Zurz Z, Chau BH, Schlessinger A, Bonomi M, Harries W, Sali A, Johri AK, Stroud RM. Crystal structure of a eukaryotic phosphate transporter. Nature. 2013 Apr 25;496:533-536.