Structural results of importance to cancer research came from the group of Seth Rubin at the University of California at Santa Cruz, who solved the structure of Cks, a conserved regulator of cyclin-dependent kinase (CDK). Clarifying the underlying mechanisms and cellular contexts of Cks function is critical because Cks is essential for proper cell growth, and its overexpression has been linked to cancer. The group observed that budding-yeast Cks associates with select phosphorylated sequences in cell-cycle-regulatory proteins. The 2.9-? crystal structure of Cks bound to a phosphorylated CDK substrate revealed the molecular interactions responsible for specificity. The structural details motivated the mutational analysis that demonstrated that Cks enhances CDK activity in response to phosphorylation of specific priming sites. The structural work was a basis for characterizing the biochemical function of Cks and provided detailed molecular insights into an important mechanism of cell cycle regulation.
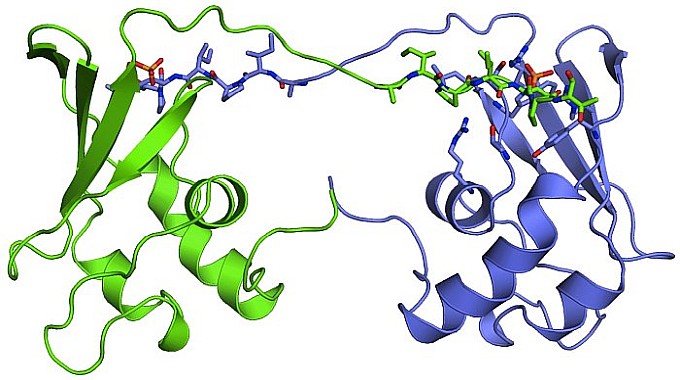 |
Figure: Peptide-swapped Cks. |
Citation: McGrath, DA, Balog, ERM, K?ivom?gi, M, Lucena, R, Mai, MV, Hirschi, A, Kellogg, DR, Loog, M, Rubin, SM. Cks confers specificity to phosphorylation-dependent CDK signaling pathways, Nat. Struct. Mol. Biol. 20 (12), 1407-1414 (2013). DOI: 10.1038/nsmb.2707