Human adenoviruses (HAdVs) are large (~150 nm in diameter, 150 MDa) nonenveloped double-stranded DNA viruses that cause respiratory, ocular and enteric diseases. Because of the broad cell tropism and ease of genome manipulation, replication-deficient or conditionally replication-deficient HAdVs are being used in the clinic as potential vaccine and gene delivery vectors. The capsid shell of an adenovirus (Ad) comprises multiple copies of three major capsid proteins (MCPs: hexon, penton base and fiber) and four minor/cement proteins (IIIa, VI, VIII and IX) that are organized with pseudo T=25 icosahedral symmetry. In addition, six other proteins are encapsidated along with the 36-kb dsDNA genome. The structures of individual MCPs and their organization within the capsid shell are known from prior X-ray crystallography and cryoEM studies. However, the details of structures of the glue (cement) proteins, which are essential for formation of Ad virions, as well as their locations in the capsid were unclear and have become source of contention because previous assignments were inconsistent biochemical results. Employing X-ray crystallographic analysis of data collected at GM/CA@APS, Reddy and Nemerow resolved structures of all the cement proteins within the capsid, revealing an overall structure that is consistent with the biochemical observations over the past 40 years. Of note, the revised structures of the cement proteins, protein VI in particular, provided insights into the requirement for a final step of capsid maturation during virus infection. This involves the cleavage of N-terminal peptide of protein VI by the viral protease, thereby enabling the release of a membrane lytic domain, which has been implicated in the endosome lysis and release of virions into the cytoplasm. Finally the structures and organization of the cement proteins elucidated by crystal structure analysis of HAdV, the largest biomolecule (~950 Å in diameter) yet studied by X-ray crystallography, represent a consensus model for understanding adenovirus assembly and disassembly.
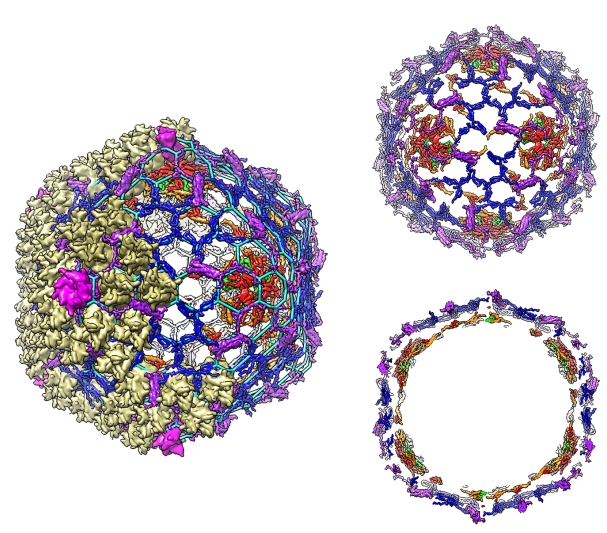 |
Figure: Organization of adenovirus cement proteins (CmPs) with respect to the major capsid proteins (left): hexon (khaki) and penton (magenta). The cement protein scaffold (top right) and its cross-section (bottom right) show that CmPs are organized in two layers. The outer layer comprises proteins IIIa (purple) and IX (blue), which form a hexagonal lattice (left; cyan), while the inner layer consists of proteins V (green), VI (red) and VIII (orange). |
Citation: Vijay S. Reddy, Glen R. Nemerow, "Structures and organization of adenovirus cement proteins provide insights into the role of capsid maturation in virus entry and infection," Proc. Natl. Acad. Sci. USA 111 (32), 11715-11720 (2014). DOI: 10.1073/pnas.1408462111 [ Note also: Reply to Campos: Revised structures of adenovirus cement proteins represent a consensus model for understanding virus assembly and disassembly Proc. Natl. Acad. Sci. USA 2014 111 (43) E4544-E4545 ]