 |
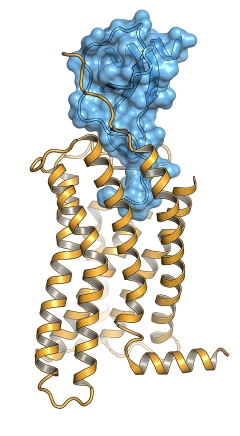
Figure: Surface representation of the chemokine ligand, CX3CL1 (blue), bound to the viral chemokine GPCR US28 (orange). |
Given the importance of chemokines in various pathologies and in drug development, Chris Garcia's group at Stanford University solved the structure of a different GPCR:protein complex -- an activated cytomegalovirus GPCR, US28, bound to its human chemokine ligand, CX3CL1. In this case, US28 is a viral GPCR that binds a human chemokine to subvert the host immune system. The structure revealed how the N-terminus of the chemokine enters the binding pocket of the receptor in the center of the 7-helix bundle. The N-terminus of the receptor binds to a conserved groove on the surface of the chemokine. The structure also revealed a unique amino acid network that destabilizes the receptor's inactive state, contributing to its constitutive activity. These findings offer insights into GPCR ligand binding and receptor activation that advance the understanding of mechanisms of immune evasion and will guide rational drug design.
Citation: John S. Burg, Jessica R. Ingram, A.J. Venkatakrishnan, Kevin M. Jude, Abhiram Dukkipati, Evan N. Feinberg, Alessandro Angelini, Deepa Waghray, Ron O. Dror, Hidde L. Ploegh, K. Christopher Garcia, "Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor," Science 347, 1113-1117 (2015). DOI: 10.1126/science.aaa5026