In addition to the Sec translocon, bacteria also employ dedicated transport systems to secrete specific cargo proteins for the benefit of their survival in a competitive environment, such as for quorum sensing and for secreting antimicrobial polypeptides to decrease competition from other bacteria. The simplest Sec-independent pathway is a dual-function ATP-binding cassette (ABC) transporter that processes and secretes polypeptides. ABC transporters are a large family of membrane proteins that harness the energy from ATP hydrolysis to drive substrate translocation. All ABC transporters share a conserved architecture of two transmembrane domains (TMDs) and two nucleotide-binding domains (NBDs), but the transporters involved in protein secretion are unique among ABC transporters in that they contain additional cysteine-protease peptidase domains, which are essential for substrate processing. Using beamlines at GM/CA@APS and at NE CAT, researchers from Jue Chen's laboratory (Rockefeller University) determined the structure of a molecular pump that controls the passage of proteins through a bacterial cell membrane, offering new insight into the mechanics that allow bacteria to manipulate their environments. This pump, called PCAT for peptidase-containing ATP-binding cassette transporter, is composed of a single protein, capable of recognizing and processing its cargo, and then burning ATP to pump that cargo out of the cell. The structure of PCAT was determined in two conformational states. In the absence of ATP, the transmembrane domains enclose a large, water-filled central channel, a natural environment for a hydrophilic protein. Two side openings into this channel were guarded by the peptidase domain, acting as a sort of ticket taker. When ATP is present, the cytoplasmic entrances close, the peptidase domains dissociate, and the ATP-binding domains come together. These structures lead to a hypothesis that energy from ATP allows PCAT to change conformation in such a way that it pushes its cargo out.
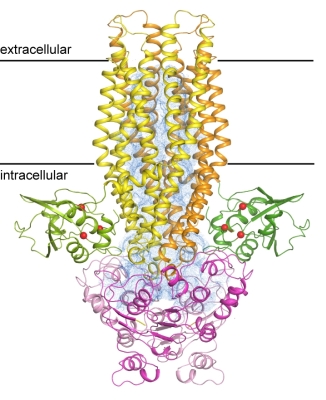 |
Figure: Structure of the PCAT transporter in a nucleotide-free state. The peptidase domain is shown in green, where the catalytic residues are indicated as red balls. The transmembrane domain is in yellow, and the nucleotide-binding domain is shown in magenta. The two subunits are distinguished by different shades. The transmembrane cavity is shown as a blue mesh. |
Citation: Lin, D. Y., Huang, S., and Chen, J. (2015) Crystal structures of a polypeptide processing and secretion transporter, Nature 523, 425-430.