The structure of a membrane-related protein important in cholesterol metabolism was studied by John Tesmer's group and collaborators at the University of Michigan, who determined several high-resolution structures of lysosomal phospholipase A2 (LPLA2) in complex with irreversible inhibitors and a low-resolution structure of lecithin:cholesterol acyltransferase (LCAT). These are the first X-ray structures of these key lipid-metabolizing enzymes. LPLA2 is a lysosomal enzyme involved in the lung-surfactant metabolism and its inhibition by cationic lipophilic drugs is predicted to cause drug-induced phospholipidosis. LCAT is important in reverse cholesterol transport by catalyzing the esterification of cholesterol in HDL particles, and thus is being investigated for its role in preventing atherosclerosis. Additionally, missense mutations in the LCAT gene cause two genetic diseases, fish eye disease and familial LCAT deficiency. Structures of LPLA2 and LCAT allowed the investigators to understand the mechanisms of substrate recognition, membrane interaction, and activation by intermolecular cues such as by HDL. Such knowledge will be useful in developing small molecule activators and biotherapeutics to treat genetic and cardiovascular diseases.
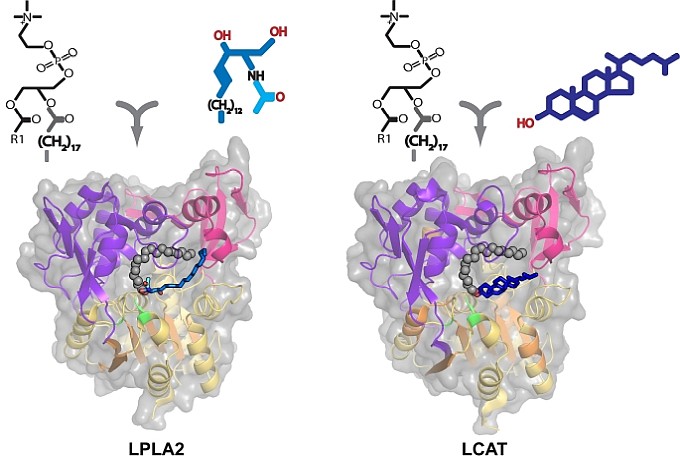 |
Figure: Reactions and structures of acylated LPLA2 (left) and LCAT (right) in complex with their preferred acyl-group acceptors, N-acetyl sphingosine and cholesterol, respectively. |
Citation: Alisa Glukhova, Vania Hinkovska-Galcheva, Robert Kelly, Akira Abe, James A. Shayman, John J.G. Tesmer , "Structure and function of lysosomal phospholipase A2 and lecithin:cholesterol acyltransferase," Nat. Commun. 6, 6250-1 (2015). DOI: 10.1038/ncomms7250