 |
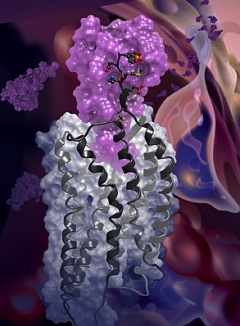
Figure: Structure of the Chemokine receptor, CXCR4 (gray), complexed to the viral chemokine, vMIPII (magenta). |
The group of Tracy Handel (University of California, San Diego), in collaboration with the Stevens group, determined the structure of a GPCR:protein complex between the chemokine receptor, CXCR4, and a viral chemokine, vMIP II. Chemokines and their receptors control cell migration during development, immune system responses, and in numerous diseases, including inflammation and cancer. CXCR4 is one of two co-receptors that facilitate entry of HIV into host immune cells. vMIP II from Kaposi's sarcoma-associated herpesvirus is a chemokine that helps the virus to escape the host immune system. The structural basis of receptor:chemokine recognition has been a long-standing unanswered question due to the challenges of structure determination for membrane protein complexes. vMIP-II was chosen for structural studies because it is a high-affinity antagonist of CXCR4, and, as a ligand for both CC and CXC chemokine receptors, was expected to provide insight into ligand recognition specificity. The structure helped to rationalize a large body of mutagenesis data and together with modeling provided insights into CXCR4 interactions with its endogenous ligand CXCL12, its ability to recognize diverse ligands, and the specificity of CC and CXC receptors for their respective chemokines. The growing number of chemokine receptor structures with different ligands opens possibilities for rational design of ligands that have improved inhibition profiles and mechanisms of action.
Citation: Qin L, Kufareva I, Holden LG, Wang C, Zheng Y, Zhao C, Fenalti G, Wu H, Han GW, Cherezov V, Abagyan R, Stevens RC, Handel TM. Structural biology. Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine. Science. 2015 Mar 6;347(6226):1117-22. doi: 10.1126/science.1261064.