Shelagh Ferguson-Miller's group at Michigan State University determined high-resolution crystal structures of an alpha-helical membrane protein, the Rhodobacter homolog of mitochondrial translocator protein 18 kDa (TSPO), as well as a mutant form that mimics a human disease-related polymorphism. TSPO is an ancient conserved protein whose functions in bacteria and in higher eukaryotes are yet to be clearly defined. It was first identified as a mitochondrial outer membrane protein that binds benzodiazepine drugs, but distinct from the GABAA receptor of the central nervous system. Originally called the peripheral benzodiazepine receptor (PBR), it was renamed the translocator protein or TSPO, in recognition of its far-reaching phylogeny and mounting evidence of involvement in a number of complex normal and abnormal cellular processes including cholesterol transport, porphyrin transport, inflammation and tumor progression, as well as in Parkinson's and Alzheimer's diseases. A recently identified single polymorphism in human TSPO (Ala147Thr) is associated with anxiety-related diseases, making it a potential target for anxiolytic drugs. There is also widespread use of TSPO ligands for imaging of brain damage, due to the consistently high expression of TSPO in regions of neuroinflammation. TSPO was also discovered in photosynthetic bacteria as a regulator of the transition between respiration and photosynthesis, an activity that appears related to its ability to bind and translocate porphyrins. Importantly, a knock-out of bacterial Rhodobacter sphaeroides can be complemented by rat TSPO, indicating a highly conserved molecular function. The TSPO structure is a 5 transmembrane helical bundle, unlike most outer membrane proteins, which are beta-barrels. The structures also show significant differences between the wild-type and the Ala139Thr mimic of the human polymorphyism. The mutant has a more closed conformation and a reduced affinity for cholesterol and porphyrin, consistent with the human phenotype. A closely associated dimer was found in three different crystal forms, implying functional significance, but the tight dimer interface does not suggest a role in transport as had been proposed. An endogenous porphyrin ligand was also resolved in the X-ray structures, but no drug or cholesterol molecules were observed, leaving many unanswered questions regarding the function of this ancient multifaceted protein.
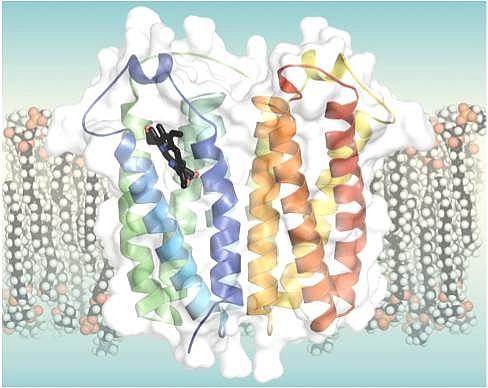 |
Figure: Crystal structure of RsTSPO (4UC1) with an endogenous ligand, a porphyrin (black), in the central cavity of one monomer of the dimeric protein, represented in a simulated membrane. |
Citation: Fei Li, Jian Liu, Yi Zheng, R. Michael Garavito, Shelagh Ferguson-Miller, "Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism," Science 347, 555-558 (2015). DOI: 10.1126/science.1260590