The group of Arvin Dar, Neil Dhawan, and Alex Scopton has identified the first small-molecule antagonists of the human scaffold protein Kinase Suppressor of Ras (KSR). KSR is a dynamic regulator of oncogenic Ras - the most frequently mutated gene within human cancers. Because of KSR's role in Ras signaling, it had long been considered a possible drug target. The group was able to identify a novel class of small-molecule compounds that bind directly to KSR to antagonize critical protein-protein interactions within the Ras signaling pathway. Structures of KSR bound to a lead compound (KSRi) and the Ras effector MEK suggest a mechanism whereby small-molecule binding induces a previously unrecognized inactive-state conformation in KSR. The structures also demonstrate the potential of targeting KSR in human cancers, providing a template for future drug-discovery efforts.
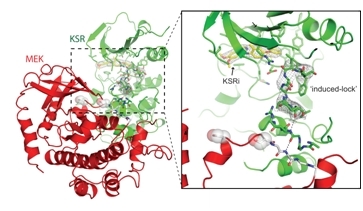 |
The figure shows small-molecule stabilization of the KSR inactive state via binding of a small-molecule compound (KSRi). |
Dhawan NS, Scopton AP, Dar AC, "Small molecule stabilization of the KSR inactive state antagonizes oncogenic Ras signaling," Nature 537, 112-116 (2016). DOI: 10.1038/nature19327