Andrew Kruse and his group determined the structure of the enigmatic human σ1 receptor. The transmembrane receptor is located in the endoplasmic reticulum (ER) membrane and is implicated in a variety of neurological disorders including depression, drug addiction, and neuropathic pain. It has also been linked to amyotrophic lateral sclerosis (ALS). Although first identified about 50 years ago by its ability to bind radiolabelled opioids, σ1 is not considered a true opioid receptor, and it is not a GPCR. Until now, the structure has been elusive and basic questions, such as the number of transmembrane domains, remained unanswered. The Kruse lab grew crystals in the lipid cubic phase (LCP), and used data from GM/CA@APS beamlines 23ID-D and 23ID-B and from NE CAT to determine the structure. The trimeric receptor has a triangular architecture with a single transmembrane helix in each protomer. An extensive hydrophobic surface on the C-terminal extrinsic domain presumably associates with the cytosolic surface of the ER membrane. The extrinsic domain has a cupin-like β-barrel fold with a large, hydrophobic ligand-binding cavity. The ligand-binding cavity shows remarkable plasticity in ligand recognition, as seen in complexes with two dissimilar ligands, which bind in similar positions. In addition, the structure showed the structural basis for receptor destabilization resulting from an ALS-associated mutation. Knowledge of the σ1 receptor structure opens the door to detailed investigation of its role in both normal and pathophysiological conditions.
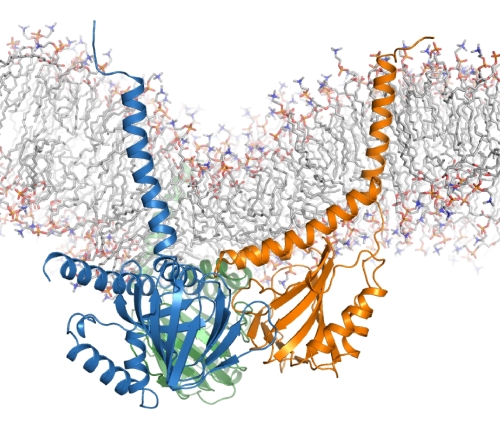 |
Figure: Structure of the trimeric human σ1 receptor, showing the N-terminal transmembrane helix and the C-terminal ligand-binding domain of the blue, orange and green monomers. |
Citation: Schmidt, H.R., Zheng, S., Gurpinar, E., Koehl, A., Manglik, A., Kruse, A.C. (2016) Crystal structure of the human σ1 receptor, Nature, 532, 527-530.