 |
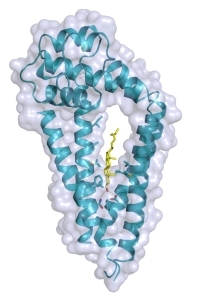
The crystal structure of CD81 revealed a cholesterol binding site at the center of the protein's four transmembrane helices |
The Blacklow and Kruse labs at Harvard Medical School determined the structure of human CD81, the first example of a full length tetraspanin to be characterized structurally. The tetraspanins are a poorly understood family of membrane proteins that play diverse biological roles, particularly in the function of the immune system. In particular, the tetraspanin CD81 has an essential role in B cell development and function. The crystal structure of CD81 revealed a cholesterol binding site at the center of the protein's four transmembrane helices. Biochemical and cell biological assays, together with molecular dynamics simulations in the Dror lab at Stanford University, revealed a possible regulatory role for cholesterol binding, which may control CD81 conformation and function. The high sequence conservation of the transmembrane region suggests this may be a general feature of many or all members of the tetraspanin family - a subject for future investigation.
Zimmerman B, Kelly B, McMillan BJ, Seegar TCM, Dror RO, Kruse AC, Blacklow SC, "Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket," Cell 167, 1041-1051 (2016). DOI: 10.1016/j.cell.2016.09.056