 |
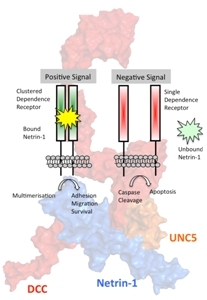
Figure: Surface rendering of the netrin-1/UNC5H complex and schematic diagram of the chemoattraction and chemorepulsion functions of netrin-1. |
The group of Joerg Stetefeld determined an important structure in the battle against cancer. Along with collaborators at the University of Cologne and the CNRS in Lyon, the investigators aimed to unravel the molecular mechanism of the Netrin-1/UNC5H interaction. Netrin-1 is a bifunctional guidance cue that regulates the adhesion, migration and survival of cells through its interaction with dependence receptors and orchestrates chemoattraction (via NEO1/DCC), as well as chemorepulsion (via UNC5H). Netrin-1 is up-regulated in a fraction of human cancers as a mechanism to block the pro-apoptotic activity of the UNC5H receptors. Stetefeld and colleagues identified in atomic detail the UNC5H-binding epitope of netrin-1 and identified an antibody targeting netrin-1 and disrupting the UNC5H interaction. The antibody has therapeutic potential both in solid tumors and in hematological malignancies, and clinical trials will begin this year.
Citation: Grandin M, Meier M, Delcros JG, Nikodemus D, Reuten R, Patel TR, Goldschneider D, Orriss G, Krahn N, Boussouar A, Abes R, Dean Y, Neves D, Bernet A, Depil S, Schneiders F, Poole K, Dante R, Koch M, Mehlen P, and Stetefeld J. Structural decoding of the netrin-1/UNC5 interaction and its therapeutical implications in cancers. (2016) Cancer Cell 29, 173-185.