 |
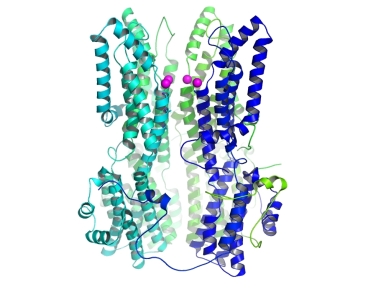
The structure of a calcium-independent BEST1 mutant (I76A, F80A, F84A). One of the five subunits is removed to show the pore, and the magenta spheres are chloride atoms just above the pore entrance. |
The group of Stephen Long at the Memorial Sloan Kettering Cancer Center used X-ray crystallography at GM/CA@APS and at the NSLS to study the function of the bestrophin (BEST1) ion channel. BEST1 is a calcium-activated chloride channel found in the plasma membrane of a variety of cell types. Mutations in BEST1 can cause macular degeneration. Calcium binding to the cytoplasmic region of the BEST1 channel causes channel opening. This work identifies a calcium-dependent channel gate consisting of three conserved hydrophobic amino acids. The researchers determined a high-resolution structure of a gate mutant using data from 23ID-D to show how widening the pore in the gate region leads to calcium-independent activity.
Vaisey G, Miller AN, Long SB, "Distinct regions that control ion selectivity and calcium-dependent activation in the bestrophin ion channel," Proc. Natl. Acad. Sci. USA 113 (47), E7399-E7408 (2016). DOI: 10.1073/pnas.1614688113