 |
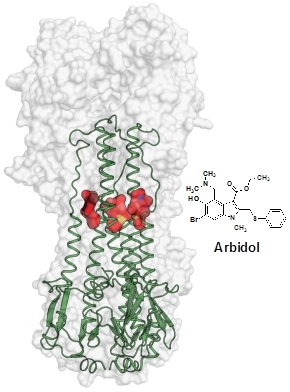
The structure demonstrates that this drug targets a highly-conserved pocket in the HA stem region |
The group of Ian Wilson at The Scripps Research Institute determined crystal structures of hemagglutinin (HA) from pandemic (H3N2) and emerging strains (H7N9) of influenza virus in complex with a general antiviral drug, Arbidol. Arbidol is used as anti-influenza drug in Russia and China, and is currently in stage-four clinical trials in the United States. The structures demonstrated that this drug targets a highly-conserved pocket in the HA stem region. Arbidol acts as a molecular glue that stabilizes the HA trimer and prevents pH-induced conformational changes in the endosome that would normally lead to fusion of viral and host cell membranes to initiate influenza infection. As influenza strains have acquired resistance to all approved drugs worldwide against current influenza targets, structural information on the Arbidol-HA interaction provides valuable insights for development of potent small-molecule therapeutics that target the influenza virus hemagglutinin.
Kadam RU, Wilson IA, "Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol," Proc. Natl. Acad. Sci. USA 114 (2), 206-214 (2017). DOI: 10.1073/pnas.1617020114