Throughout the world, HIV occurs in different strains or clades, each with a distinct variant of the surface glycoprotein (Env), which is the sole neutralization target of antibodies. Env forms a heavily glycosylated trimer of gp120/gp41 heterodimers that facilitates fusion of the virus membrane and the host cell membrane. In 2013, using data collected at GM/CA beamlines, Wilson's group published the structure of the envelope glycoprotein (Env) from Clade A. However, Clade C is responsible for the majority of infections worldwide, but Env from Clade C is notoriously unstable. Recently, using glycine substitutions to stabilize the pre-fusion state and data from multiple crystals, Wilson's group and collaborators determined the structure of Clade C Env to 3.9-Angstrom resolution. The structure led to the design of cross-clade immunogens, which in additional studies were shown to illicit neutralizing antibodies in non-human primates, and may lead to an effective vaccine for humans.
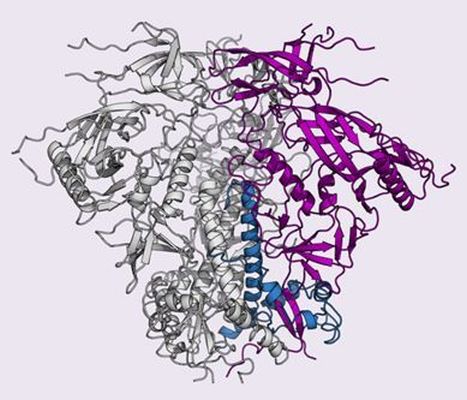 |
Figure: Structure of Env trimer from HIV Clade C. |
Citation: Guenaga J, Garces F, de Val N, Stanfield RL, Dubrovskaya V, Higgins B, Carrette B, Ward AB, Wilson IA, Wyatt RT, Glycine Substitution at Helix-to-Coil Transitions Facilitates the Structural Determination of a Stabilized Subtype C HIV Envelope Glycoprotein, Immunity 46, 792 (May 16, 2017). DOI: 10.1016/j.immuni.2017.04.014